Belousov–Zhabotinsky reaction facts for kids
The Belousov–Zhabotinsky reaction (often called BZR or BZ) is a special type of chemical reaction that acts like a chemical oscillator. This means it changes back and forth in a repeating pattern, almost like a pendulum. It's often used to show how a chaotic system works, where small changes can lead to big, surprising results.
The BZR is actually made up of several chemical reactions happening one after another. What makes it so unusual is that it shows chemical oscillation, meaning its appearance or properties change in a cycle. When it was first discovered, people thought it was a mistake or a problem with the experiment. This was because they believed that, according to the Second law of thermodynamics, a system that is messy shouldn't become organized on its own without adding energy. However, this law only applies to a "closed system" (where nothing can enter or leave). The BZ reaction is not a closed system, so it can create these amazing patterns.
These reactions are very important in science. They show that chemical reactions don't always have to settle down into a calm, balanced state. The BZ reaction stays active and far from balance for a long time. Because of this, it's a great example of how living things, which are always active and changing, work at a chemical level. Scientists also use mathematical models to understand the BZ reactions, which helps them learn more about complex systems.
A key feature of the BZ reaction is its "excitability." This means that if you give it a little push or trigger, it can start to form patterns in what was a calm liquid. Some other "clock reactions," like the Briggs–Rauscher reaction, can also show this "self-organizing" behavior. This means they can create their own patterns, sometimes even with the help of light, especially when using a special chemical called a catalyst like tris(bipyridine)ruthenium(II) chloride.
History of the BZ Reaction
Similar chemical processes were known even in the 1800s. A scientist named Wilhelm Ostwald wrote about them in detail around 1899 and 1900. He described how chemicals like Chrome in Hydrochloric acid and iron in Nitric acid could show these repeating changes.
The Belousov–Zhabotinsky reaction itself was found by accident in the 1950s by Boris Pavlovich Belousov. Later, Ilya Prigogine won the Nobel Prize in Chemistry in 1977 for his work on systems that are far from a balanced state. The Belousov–Zhabotinsky reaction was a key part of his research. Belousov himself was honored with the Lenin Prize in 1980, after he had passed away. The Lenin Prize was the highest award for scientific achievements in the Soviet Union.
How the BZ Reaction Looks
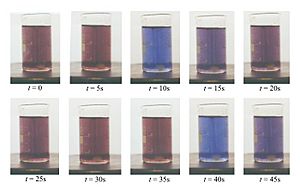
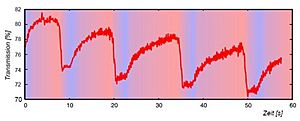
The BZ reaction is famous for its beautiful, changing patterns. You can often see waves or spirals of different colors forming and moving through the liquid. These patterns happen because the chemicals are constantly changing their state, then changing back, and then changing again. It's like a chemical dance that keeps repeating. Scientists can even measure how the light passes through the reaction to see these changes.
Images for kids
-
.
Computer simulation of the Belousov–Zhabotinsky reaction
See also
 In Spanish: Reacción de Beloúsov-Zhabotinski para niños
In Spanish: Reacción de Beloúsov-Zhabotinski para niños
 | Delilah Pierce |
 | Gordon Parks |
 | Augusta Savage |
 | Charles Ethan Porter |