Carbon–hydrogen bond activation facts for kids
Carbon–hydrogen bond activation, or C-H activation, is a special type of chemical reaction that breaks a strong connection between a carbon atom and a hydrogen atom. This reaction often uses organometallic compounds, which are molecules that have a metal atom connected to carbon atoms.
In C-H activation, a hydrocarbon molecule (which is made of carbon and hydrogen) gets close to a metal atom. The metal helps to weaken and then break the bond between the carbon and hydrogen. This process is different from other reactions because the carbon-hydrogen part stays connected to the metal atom as the bond breaks.
Chemists study C-H activation because for a long time, they thought that C-H bonds were very hard to break. Now, both scientific theories and experiments show that these bonds can be broken. This happens when a nearby metal atom changes how the electrons are shared in the C-H bond.
A lot of chemical research focuses on making new chemicals and catalysts that can do C-H activation. The main goal is to turn cheap and common substances like alkanes (like methane or propane) into more valuable organic compounds that can be used to make medicines, plastics, or other useful materials.
History of C-H Activation
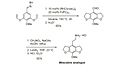
Historians say that Otto Dimroth found the first C-H activation reaction in 1902. He reported that benzene, a type of hydrocarbon, reacted with mercury(II) acetate. However, some scientists do not fully agree that this was the first true C-H activation.
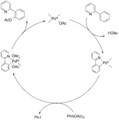
Later, scientists Goldman and Goldberg explained that C-H activation is similar to how hydrogen-hydrogen (H-H) bonds are broken. Both can happen through processes called electrophilic or oxidative addition.
The first reaction that was widely accepted as a true C-H activation was reported by Joseph Chatt in 1965. In his experiment, a ruthenium atom, which was attached to other molecules called ligands, inserted itself into a C-H bond of naphthalene. This showed clearly that a metal could directly break and interact with a C-H bond.
See also
- Oxidative coupling of methane
Images for kids
See also
 In Spanish: Activación del enlace C-H para niños
In Spanish: Activación del enlace C-H para niños
 | Valerie Thomas |
 | Frederick McKinley Jones |
 | George Edward Alcorn Jr. |
 | Thomas Mensah |