Casein facts for kids
Casein (pronounced KAY-seen) is a special type of protein found in milk. It makes up about 80% of the proteins in cow's milk. Human milk has less casein, while milk from sheep and buffalo has more.
Casein is used in many ways. It's a main ingredient in cheese, and it's also added to many foods. A common form of casein is called sodium caseinate. In milk, casein forms tiny clumps called casein micelles. These are like little packages of protein.
Casein is a great food source because it provides important things like amino acids (the building blocks of proteins), some carbohydrates, and two key minerals: calcium and phosphorus.
Contents
What is Casein Made Of?
Casein proteins have a lot of a specific amino acid called proline. This makes it hard for them to form common protein shapes. They also don't have certain strong bonds called disulfide bridges. Because of this, casein doesn't have a very fixed shape.
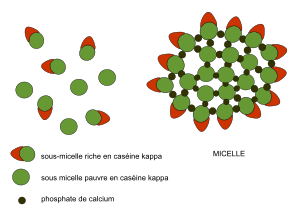
Casein doesn't mix well with water. That's why it's found in milk as tiny particles called micelles. These micelles are like small, round clusters. The parts of casein that like water are on the outside of these clusters. The inside of a casein micelle holds a lot of water. The casein proteins in these micelles are held together by calcium and by their water-avoiding parts.
Casein has a negative charge in milk because milk's pH is 6.6. Pure casein doesn't dissolve in water. However, it can mix easily in weak alkali solutions, like those with sodium oxalate.
An enzyme called trypsin can break down casein. This process can be used to make a type of organic adhesive or glue.
How Casein is Used
Casein in Paint
Casein paint is a type of paint that dries quickly and can be mixed with water. Artists have used casein paint for a very long time, even since ancient Egyptian times! It was a popular choice for illustrators until the 1960s when acrylic paint became more common. Scenic painters, who paint backgrounds for plays and movies, still use casein paint.
Casein in Glue
Glues made from casein are created using casein, water, and special chemicals called alkalis (like hydrated lime and sodium hydroxide). To make the glue, milk is first skimmed to remove the fat. Then, the milk is soured to make the casein separate into milk curd. This curd is washed to remove the liquid part (called whey) and then pressed to squeeze out the water. Sometimes it's even dried into a powder.
The casein is then mixed with alkalis to create the glue. Different mixes of alkalis give the glue different properties. Preservatives might also be added.
Casein glues were very popular for woodworking, even for building airplanes, like the de Havilland Albatross airliner in 1939. They are also used in making transformers because they allow oil to pass through.
Famous glues like Elmer's Glue-All and Elmer's School Glue were originally made from casein. This was partly because it was non-toxic and also very cheap to use. Later, in the late 1900s, companies started using synthetic materials like PVA instead of casein in their popular glues.
Even though synthetic glues are now more common, casein-based glues are still used for special purposes, such as laminating fireproof doors and labeling bottles. Casein glues become thinner when they get warmer, which makes it easy to apply thin layers quickly to labels on a production line.
Casein in Food
Many foods, coffee creamers, and toppings contain different types of caseinates. Sodium caseinate is often used as a food additive to help stabilize processed foods. Companies might also use calcium caseinate to add more calcium and reduce sodium in their products.
| Product | Caseinate % | What it Does |
|---|---|---|
| Meat | 2–20 | Helps with texture and adds nutrition |
| Cheese | 3–28 | Forms the cheese structure, binds fat and water |
| Ice Cream | 1–7 | Improves texture and keeps it stable |
| Whipped toppings | 2–11 | Helps keep fat stable |
| Pasta | 2–18 | Improves texture, adds nutrition, and taste |
| Baked goods | 1–15 | Helps bind water |
Casein is often used in powders that need to mix quickly with water, like coffee creamers or instant cream soups. It is also believed to help neutralize the spicy feeling from chili peppers.
Casein in Cheesemaking
Cheese is made from the proteins and fat in milk, usually from cows, buffalo, goats, or sheep. Cheese is made by causing the casein in milk to clump together. This process separates the milk into solids and liquids.
First, the milk is made more acidic. Then, an enzyme called rennet is added. Rennet traditionally comes from the stomachs of calves, but today it's often made using special microorganisms. The rennet helps the casein micelles clump together. The solid clumps are then separated from the liquid whey and pressed into the final cheese shape.
Unlike many proteins, casein does not clump together when heated. When milk clots to make cheese, the rennet acts on a specific part of the casein called κ-casein. This makes the casein micelles unstable and causes them to form a clot. When casein is clotted with rennet, it's sometimes called paracasein.
Casein in Protein Supplements
One cool thing about casein is that it can form a gel or clot in your stomach. This makes it very good at delivering nutrients slowly. The clot slowly releases amino acids into your bloodstream over several hours. Casein is often sold as hydrolyzed casein, which means it has been partly broken down by an enzyme like trypsin.
Casein in Plastics and Fiber

Some of the very first plastics were made from casein. One famous type was called galalith, which was often used to make buttons. You can also make fiber from casein. A fabric called Lanital (known as Aralac in the United States) was made from casein fiber and was popular in Italy in the 1930s. Newer ideas, like QMILK, are using casein fiber in more advanced ways for modern fabrics.
Casein in Medicine and Dentistry
Compounds made from casein are used in products that help rebuild tooth enamel. They help to stabilize a substance called amorphous calcium phosphate (ACP) and release it onto tooth surfaces, which can help teeth become stronger.
Casein in Nanotechnology
Casein proteins are being looked at for use in nanomaterials. This is because they are easy to get from milk and can naturally form tiny, organized structures called amyloid fibrils.
See also
 In Spanish: Caseína para niños
In Spanish: Caseína para niños
 | Kyle Baker |
 | Joseph Yoakum |
 | Laura Wheeler Waring |
 | Henry Ossawa Tanner |