Cathode ray facts for kids
A cathode ray is like a super-fast stream of tiny particles called electrons. Imagine a beam of light, but instead of light, it's made of these tiny electrons moving very quickly! You can see them inside special glass tubes called vacuum tubes. They are called "cathode rays" because they shoot out from the negative part of the tube, which is called the cathode.
These rays were first noticed way back in 1869 by a German scientist named Johann Wilhelm Hittorf. Later, in 1876, another scientist, Eugen Goldstein, gave them their name, Kathodenstrahlen, which means cathode rays.
In 1897, a British physicist named J. J. Thomson made a big discovery. He figured out that these cathode rays were actually made of tiny, negatively charged particles. He called these particles "electrons." This was a huge step in understanding how electricity and matter work!
Old-fashioned television sets and computer monitors used something called a Cathode ray tube (CRT). These CRTs used a focused beam of electrons (cathode rays) to create the pictures you saw on the screen. Tiny electric or magnetic fields would move the electron beam around very fast to draw the image.
Cool Facts About Cathode Rays
Here are some interesting things about cathode rays:
- They travel in a straight line, like a laser beam.
- They carry a negative electric charge.
- They are made of real particles, not just energy.
- You can bend them with magnets.
- Their properties, like their charge and mass, are always the same.
- They always travel from the cathode (negative side) to the anode (positive side) inside the tube.
- What the tube is made of, or what gas is inside, doesn't change how the rays behave.
- They can make gas atoms lose electrons, a process called ionization.
- They move fast, but not as fast as light.
- If they hit something, they can make it warm up.
- They can even go through very thin pieces of metal, like aluminum foil.
- They can make special materials called phosphors glow brightly.
Images for kids
-
A beam of cathode rays in a vacuum tube bent into a circle by a magnetic field generated by a Helmholtz coil. Cathode rays are normally invisible; in this demonstration with a Teltron tube, enough gas has been left in the tube that the gas atoms luminesce when struck by the fast-moving electrons.
-
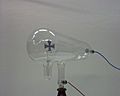
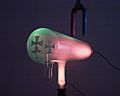
Cathode rays travel from the cathode at the rear of the tube, striking the glass front, making it glow green by fluorescence. A metal cross in the tube casts a shadow, demonstrating that the rays travel in straight lines.
See also
 In Spanish: Rayos catódicos para niños
In Spanish: Rayos catódicos para niños