Dyotropic reaction facts for kids
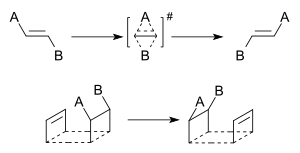
A dyotropic reaction is a special type of chemical reaction in organic chemistry. It happens when an organic compound changes its structure. During this reaction, two parts of the molecule, called substituents, move or "jump" from one place to another on the same molecule.
This reaction is a type of pericyclic reaction. It's also a valence isomerization. This means that two sigma bonds (strong chemical connections) move at the same time to new spots within the same molecule. Dyotropic reactions are important because they help scientists understand how other reactions work. They can also be very useful steps when chemists want to build large and complex molecules.
Manfred T. Reetz first described dyotropic reactions in 1971. The name "dyotropic" comes from the Greek word dyo, which means "two." The word "rearrangement" means that the reaction changes how atoms are connected within a single molecule.
There are two main kinds of dyotropic reactions:
- A type I reaction is when two moving groups switch their places with each other.
- A type II reaction is when groups move to new bonding spots, but they don't switch places with each other.
Type I Dyotropic Reactions
In type I rearrangements, the two moving groups (let's call them Y and X) are often on opposite sides of the molecule. They then swap positions. So, if you start with Y-A-B-X, you end up with X-A-B-Y.
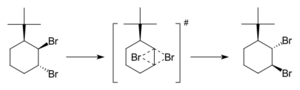
One of the first examples of this kind of reaction was found by Cyril A. Grob and Saul Winstein. They saw two bromine atoms switch places in a type of steroid molecule.
A simple example involves two bromine atoms in a molecule called 3-tert-butyl-trans-1,2-dibromohexane. When you heat it, the bromine atoms switch places. In the middle of this reaction, called the transition state, both bromine atoms are connected to both carbon atoms at the same time. This type of reaction is called "concerted" because everything happens smoothly at once. However, chemists have also found "stepwise" dyotropic reactions, where the changes happen in steps.
In organic synthesis (the process of making new organic molecules), an important type I dyotropic reaction changes certain lactones into butyrolactones. Type I dyotropic reactions can also happen with carbon-oxygen bonds. For example, heating can make two silicon groups (Si1R3 and Si2R3) swap places around a carbon-oxygen bond. This reaction can go both ways depending on the temperature. Another example is the 1,2-Wittig rearrangement. Dyotropic reactions can also involve N-O bonds (nitrogen-oxygen) or N-N bonds (nitrogen-nitrogen).
Type II Dyotropic Reactions
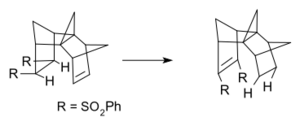
Type II rearrangements often involve two hydrogen atoms moving along a chain of carbon atoms. This type of reaction is seen in some transfer hydrogenations. Transfer hydrogenation is a process where hydrogen atoms are moved from one molecule to another.
An example of a type II dyotropic reaction is the movement of hydrogen in a molecule called syn-sesquinorbornene disulfones. Here, the hydrogen atoms move to new positions on the molecule without swapping places with each other.
See also
 In Spanish: Transposición diotrópica para niños
In Spanish: Transposición diotrópica para niños
 | Jackie Robinson |
 | Jack Johnson |
 | Althea Gibson |
 | Arthur Ashe |
 | Muhammad Ali |