Transition state facts for kids
Imagine tiny building blocks called molecules. When these molecules want to change and become new ones, they often need a little push. This push helps them get over a special, high-energy point called the transition state.
The energy needed to reach this high point is known as the activation energy. If molecules hit each other with enough energy to reach the transition state, they can then rearrange and form new molecules. At the transition state, old bonds between atoms are breaking, and new bonds are starting to form. You might see the transition state marked with a special symbol, ‡, in drawings or graphs.
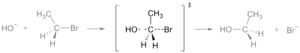
Contents
What is a Transition State?
A transition state is like the very top of a hill that molecules must climb to change. It's the moment when molecules are in a very unstable, high-energy shape, just before they become something new.
Why is it Hard to Study?
It's super tricky to study a transition state because molecules stay in this high-energy form for an incredibly short time. We're talking about femtoseconds! A femtosecond is one quadrillionth of a second – that's 0.000000000000001 seconds! Because they exist for such a tiny moment, it's hard to "catch" them and study them directly.
Transition States vs. Intermediates
It's important not to mix up transition states with intermediates.
- A transition state is a peak of energy, like the very top of a hill. It's a fleeting moment.
- An intermediate is a dip in energy, like a small valley between two hills. Molecules can stay in an intermediate form for a much longer time.
Both transition states and intermediates happen between the starting molecules (reactants) and the final molecules (products) in a chemical reaction.
Why are Transition States Important?
Studying transition states is really important for scientists to understand exactly how chemical reactions happen. This understanding helps them figure out the step-by-step process, called the reaction mechanism.
Scientists use special theories and computer programs to calculate what a transition state might look like. This field of study is part of chemical kinetics, which is all about how fast chemical reactions happen and what steps they take.
See also
 In Spanish: Estado de transición para niños
In Spanish: Estado de transición para niños
 | John T. Biggers |
 | Thomas Blackshear |
 | Mark Bradford |
 | Beverly Buchanan |

