Electrolysis of water facts for kids
Electrolysis of water is a cool process that uses electricity to break down water. Water (H₂O) is split into two different gases: oxygen (O₂) and hydrogen (H₂). Think of it like taking apart a LEGO model with an electric tool!
This method can create useful oxygen for breathing or hydrogen, which can be used as a clean fuel. However, making hydrogen this way is usually more expensive than getting it from natural gas in big factories.
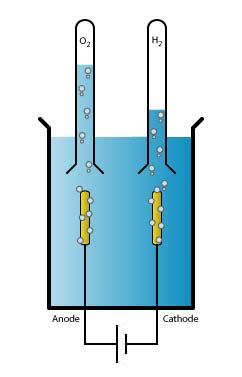
When electricity passes through water, a special chemical change happens. It's called a redox reaction. This means electrons are moved around. At one electric pole, called the cathode, a "reduction" happens. At the other pole, the anode, an "oxidation" happens. These reactions work together to split the water.
To make the process work better, sometimes a little bit of acid or a base is added to the water. This helps the electricity flow and the reactions happen more easily.
Here's the main idea of what happens:
- Water (H₂O) turns into hydrogen gas (H₂) and oxygen gas (O₂).
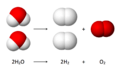
This is the overall reaction:
- 2 H₂O → 2 H₂ + O₂
It means two molecules of liquid water turn into two molecules of hydrogen gas and one molecule of oxygen gas.
Contents
A Simple Experiment You Can See
You can see electrolysis happen with simple materials. If you place two wires connected to a battery into a cup of water with some baking soda, you will see bubbles forming on the wires. These bubbles are hydrogen and oxygen gas.
A special piece of lab equipment called a Hofmann voltameter is often used to demonstrate water electrolysis in schools. It has three connected glass tubes. When electricity is passed through the water inside, it collects the hydrogen and oxygen gas in two separate tubes. This clearly shows that twice as much hydrogen is produced as oxygen.
How Electrolysis Works
Electrolysis needs a few key parts to work. You need a container for the water, two electrodes (which are like metal rods or plates), and a power source like a battery.
- Electrodes: These are the parts that touch the water and carry the electric current. One is the positive electrode (anode), and the other is the negative electrode (cathode).
- Electrolyte: This is the water, often with something added to help it conduct electricity. Pure water doesn't conduct electricity very well.
- Power Source: This provides the electricity to drive the reaction.
When the electricity flows, the water molecules break apart. Hydrogen gas bubbles form at the negative electrode, and oxygen gas bubbles form at the positive electrode. You can actually see these bubbles!
Why Is Electrolysis Important?
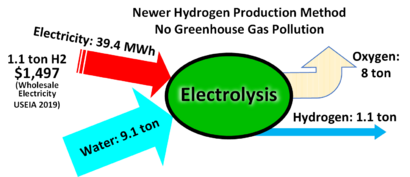
Most of the world's hydrogen is currently made from natural gas, which creates pollution. Electrolysis is important because it can create "green hydrogen" using electricity from renewable sources like solar or wind power.
- Clean Fuel: Hydrogen produced by electrolysis can be used in fuel cell cars and buses. These vehicles are very clean because they only produce water as exhaust.
- Space Exploration: The International Space Station uses electrolysis to produce breathable oxygen for the astronauts from recycled water.
- Industrial Uses: Electrolysis is used to make very pure hydrogen and oxygen. These gases are needed for making electronics, processing foods, and creating high-temperature flames for welding.
Challenges and the Future
One of the biggest challenges for water electrolysis is that it uses a lot of electricity. This can make the hydrogen it produces more expensive than hydrogen made from fossil fuels.
Scientists and engineers are working hard to make the process more efficient. Efficiency is measured by how much hydrogen you can make with a certain amount of energy. New technologies are getting better all the time. For example, an Australian company announced in 2024 a new design that is 95% efficient, meaning very little energy is wasted.
As renewable energy like solar and wind power becomes cheaper, making hydrogen through electrolysis will become a more affordable and environmentally friendly way to power our world.
Images for kids
-
A device invented by Johann Wilhelm Ritter to study the electrolysis of water.
-
A diagram showing the overall chemical equation for water electrolysis.
See also
 In Spanish: Electrólisis del agua para niños
In Spanish: Electrólisis del agua para niños
 | Georgia Louise Harris Brown |
 | Julian Abele |
 | Norma Merrick Sklarek |
 | William Sidney Pittman |