Hydron facts for kids
In chemistry, a hydron is a tiny, super important particle. Think of it as a hydrogen atom that has lost its only electron, leaving it with a positive electrical charge. While chemists often call it a proton, the official name is hydron. This is because it can be a few different types of hydrogen particles, not just the basic proton found in most hydrogen atoms.
What is a Hydron?
A hydron is essentially a positively charged ion of hydrogen. An ion is an atom or molecule that has an electrical charge because it has gained or lost electrons. Since a hydrogen atom usually has just one proton and one electron, when it loses that electron, all that's left is its positively charged nucleus.
Why Do Chemists Use "Hydron"?
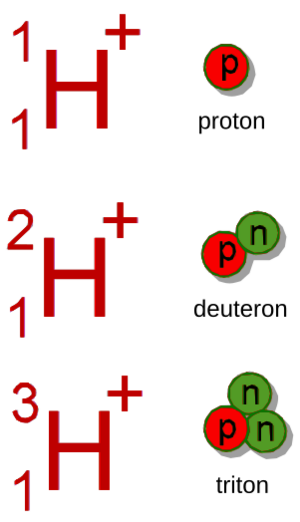
The term "hydron" is used by chemists because hydrogen can exist in different forms called isotopes. These isotopes have the same number of protons but different numbers of neutrons in their nucleus.
- A proton is the ion of regular hydrogen (hydrogen-1), which has no neutrons.
- A deuteron is the ion of deuterium (hydrogen-2), which has one neutron.
- A triton is the ion of tritium (hydrogen-3), which has two neutrons.
So, using "hydron" is a way to talk about all these positively charged hydrogen ions together, no matter which isotope they come from. This makes it easier for chemists to describe reactions.
Hydrons in Acids and Bases
In 1923, two scientists named Johannes Nicolaus Brønsted and Thomas Lowry used the idea of hydrons to explain what makes acids and bases. They came up with a simple definition:
- An acid is a substance that "donates" or gives away hydrons (protons) in a chemical reaction.
- A base is a substance that "accepts" or takes in hydrons (protons) in a chemical reaction.
This idea, known as the Brønsted-Lowry theory, helps us understand many chemical reactions, especially those that happen in water solutions. For example, when you mix an acid and a base, the acid gives a hydron to the base.