Phosphate facts for kids
Quick facts for kids Phosphate |
|
|---|---|
 |
|
|
Phosphate
|
|
| Identifiers | |
| CAS number | |
| PubChem | |
| MeSH | |
| ChEBI | CHEBI:18367 |
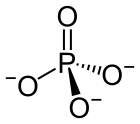
| SMILES | [O-]P([O-])([O-])=O |
| Beilstein Reference | 3903772 |
| Gmelin Reference | 1997 |
| Properties | |
| Molecular formula | |
| Molar mass | 0 g mol-1 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
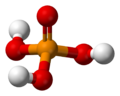
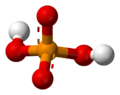
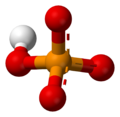
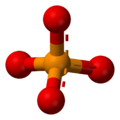
A phosphate is a special kind of salt that comes from phosphoric acid. Think of it like a building block in chemistry! Phosphates are super important in biochemistry, which is the study of chemicals in living things.
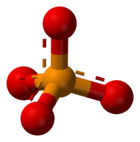
The basic formula for a phosphate is PO43-. This means it has one phosphorus atom and four oxygen atoms, with a special electrical charge. They weigh about 94.973 grams per mole. A common example is sodium phosphate. There are also different kinds of phosphates, like orthophosphate (PO43-), metaphosphate (PO32-), and pyrophosphate (P2O73-). Each has a slightly different structure.
What are Phosphates Made Of?
Every phosphate molecule has one phosphorus atom right in the middle, surrounded by four oxygen atoms. Imagine a central ball with four other balls attached to it!
Many phosphates don't dissolve easily in water. This is important for how they work in nature and in our bodies.
Images for kids
-
Phosphate mine near Flaming Gorge, Utah, US, 2008
-
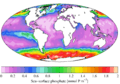
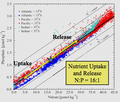
Relationship of phosphate to nitrate uptake for photosynthesis in various regions of the ocean. Note that nitrate is more often limiting than phosphate. See the Redfield ratio.
See also
 In Spanish: Fosfato para niños
In Spanish: Fosfato para niños