Phosphite ester facts for kids
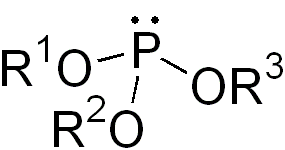
In the world of organic chemistry, a phosphite ester is a special type of chemical compound. It's also known as an organophosphite. These compounds usually have the formula P(OR)3. You can think of them as esters of a special acid called phosphorous acid (H3PO3). The simplest example is trimethylphosphite, which has the formula P(OCH3)3.
Some phosphites are also considered esters of a different form of phosphorous acid (HP(O)(OH)2). A common example of this type is dimethylphosphite, with the formula HP(O)(OCH3)2. Both kinds of phosphites are usually clear liquids with no color.
Contents
How Phosphite Esters Are Made
Phosphite esters are created in a few different ways. Let's look at the main methods.
Making Them from Phosphorus Trichloride
One common way to make phosphite esters is by mixing phosphorus trichloride (PCl3) with an alcohol.
When you use certain alcohols, like alkyl alcohols, a part of the PCl3 (a chloride ion) can react with the new phosphite. This can change the phosphite into a different compound called a dialkylphosphite, and also create an organochlorine compound. Here's a simplified way to see it:
- PCl3 + 3 C2H5OH → (C2H5O)2P(O)H + 2 HCl + C2H5Cl
Sometimes, people add a special chemical called a "proton acceptor" (often an amine base) when mixing PCl3 with alcohol. This helps create a different type of phosphite called a trialkyl derivative.
- PCl3 + 3 C2H5OH + 3 R3N → (C2H5O)3P + 3 R3NHCl
If you use alcohols like phenols (which are aromatic alcohols), you don't always need a base. These alcohols don't react with chloride in the same way. However, adding a base can still make the reaction happen faster.
Making Them by Swapping Alcohols
Phosphite esters can also be made through a process called transesterification. This means they can swap their alcohol parts when heated with other alcohols. This reaction can go both ways, which means you can make phosphites with different kinds of alcohol parts mixed together.
If you use a phosphite made from an alcohol that easily turns into a gas, like trimethyl phosphite, you can remove the gas (like methanol) by boiling it away. This helps the reaction finish completely.
Uses and Reactions of Phosphite Esters
Phosphite esters are used in many important ways, especially in industry and in making other chemicals.
How They React
Phosphites can easily react with oxygen. When they do, they change into a different type of compound called phosphate esters.
- P(OR)3 + [O] → OP(OR)3
This reaction is very important! It's why some phosphite esters are used as stabilizers in plastics. They help prevent plastics from breaking down when exposed to air.
Alkyl phosphite esters are also used in special chemical reactions. One is called the Perkow reaction, which creates vinyl phosphonates. Another is the Michaelis–Arbuzov reaction, which forms phosphonates. However, phosphites made from aromatic alcohols (aryl phosphite esters) usually don't do these reactions. That's why they are often used as stabilizers in plastics that contain chlorine, like PVC.
Phosphite esters can also act as reducing agents. This means they can help remove oxygen from other chemicals. For example, triethylphosphite can change certain hydroperoxides (chemicals with extra oxygen) into alcohols. In this process, the phosphite itself turns into a phosphate ester. This type of reaction is even used in complex chemical processes, like the Wender Taxol total synthesis, which is a way to make a cancer drug.
Using Them in Catalysis
Phosphite esters are known as Lewis bases. This means they can form special connections with different metal ions. When they do this, they create what are called coordination complexes. Some common phosphite ligands (the parts that connect to the metal) include trimethylphosphite, triethylphosphite, and triphenylphosphite.
Phosphite ligands are important parts of industrial catalysts. Catalysts are substances that speed up chemical reactions without being used up themselves. Phosphite ligands are used in processes like hydroformylation and hydrocyanation, which are used to make many useful chemicals.
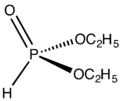
About HP(O)(OR)2 Compounds
These compounds are a bit different. They are called diorganophosphites and are related to phosphorus(V). You can think of them as di-esters of phosphorous acid ((HO)2P(O)H).
These compounds can exist in two slightly different forms that can change back and forth, a process called tautomerism. However, one form is much more common than the other:
- (RO)2POH ⇌ (RO)2P(O)H
The bond between phosphorus and hydrogen (P-H) in these compounds is very reactive. This makes them useful in reactions like the Atherton–Todd reaction and Hirao coupling. In contrast, in the tri-organophosphites we talked about earlier, the reactive part is the "lone pair" of electrons on the phosphorus atom. Like other phosphites, diorganophosphites can also undergo transesterification, meaning they can swap their alcohol parts.
See also
 In Spanish: Éster de fosfito para niños
In Spanish: Éster de fosfito para niños
- Phosphinite P(OR)R2
- Phosphonite P(OR)2R
- Ortho ester CH(OR)3
- Borate ester B(OR)3