Protein folding facts for kids
Protein folding is a super important process inside your body! It's how a protein gets its special shape. This shape is what allows the protein to do its job. Think of it like an origami artist folding a flat piece of paper into a cool crane.
Proteins start as long, wiggly chains. These chains are made of tiny building blocks called amino acids. Imagine a string of beads. This string is called a polypeptide.
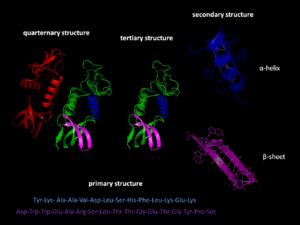
Once the polypeptide chain is made, it starts to fold itself. It doesn't need much help to do this! The amino acids in the chain are attracted to each other in specific ways. This helps the chain bend and twist into a unique 3D shape. This final, working shape is called the "native state."
Contents
Why Protein Shape Matters
A protein's shape is everything! If a protein doesn't fold correctly, it can't do its job. It's like having a key that doesn't fit the lock.
Sometimes, proteins don't fold right. When this happens, they can become inactive. They might even be harmful to the body. Scientists believe that many diseases are linked to proteins that have folded incorrectly.
For example, some allergies might happen because proteins fold in unusual ways. Our immune system then sees these odd shapes as a threat.
How Proteins Fold
Proteins go through a few steps to get their final shape:
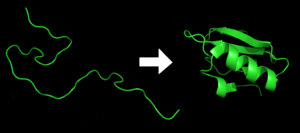
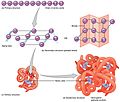
- First, a protein starts as a long, unfolded chain. This chain is made from a message copied from mRNA. It looks like a random, messy coil (see the left side of the top picture).
- Next, the amino acids in the chain start to interact with each other. They pull and push, helping the chain fold into a specific three-dimensional structure. This is the folded protein (see the right side of the top picture).
- The final shape is decided by the order of the amino acids in the chain. It's like a secret code that tells the protein how to fold.
Chaperones: Protein Helpers
Sometimes, proteins need a little help to fold correctly. That's where special helper proteins called chaperonins come in.
Chaperonins are large proteins. They act like tiny folding machines. They help other proteins fold into their right shapes after they are made.
Chaperones were first found helping parts of our DNA join up. They help form structures called nucleosomes. Nucleosomes are the building blocks for chromosomes, which carry our genetic information. Now we know chaperones help build many parts of our cells!
Images for kids
-
The alpha helix spiral formation, a common shape in proteins.
-
Steps of X-ray crystallography, a way to see protein shapes.
See also
 In Spanish: Plegamiento de proteínas para niños
In Spanish: Plegamiento de proteínas para niños
 | James Van Der Zee |
 | Alma Thomas |
 | Ellis Wilson |
 | Margaret Taylor-Burroughs |