Proton facts for kids
A proton is a tiny particle found inside every atom. Protons live in the center of an atom, called the nucleus, along with neutrons. The periodic table organizes atoms based on how many protons they have.
For example, a single hydrogen atom, which is the lightest atom, has one proton in its nucleus. An electron moves around this proton. Most of the atom's mass comes from the proton, which is almost 2,000 times heavier than an electron! Protons and neutrons together form the nucleus of all other types of atoms. The number of protons in an atom always stays the same for a specific element. This number is also called the atom's atomic number.
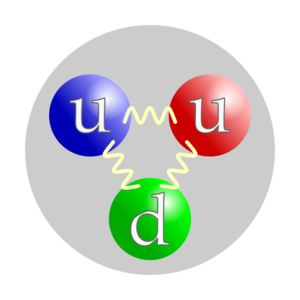
Protons are made of even smaller particles called quarks. Scientists believe a proton has three quarks: two up quarks and one down quark. Each up quark has a positive charge of +2/3, and the down quark has a negative charge of -1/3. If you add these up (+2/3 + +2/3 - 1/3), you get a total charge of +1 for the proton.
Protons are very light, but they have a specific mass. The mass of a proton is about one atomic mass unit. A neutron also has about one atomic mass unit. The size of a proton is not like a hard ball. Instead, it's more like a cloud where the quarks inside are vibrating.
History of the Proton
Scientists have thought about the idea of a basic particle that makes up all other atoms for a long time. Back in 1815, a scientist named William Prout suggested that all atoms were made of hydrogen atoms. He called these "protyles." But later, more accurate measurements showed his idea wasn't quite right.

In 1886, Eugen Goldstein found positively charged particles called "canal rays" in gases. These particles were different depending on the gas. This was different from electrons, which were always the same. Later, in 1898, Wilhelm Wien found that hydrogen ions were the lightest of these positive particles.
A big step happened in 1911 when Ernest Rutherford discovered the atomic nucleus. After this, Antonius van den Broek suggested that an element's place on the periodic table (its atomic number) was equal to the positive charge in its nucleus. Henry Moseley confirmed this idea in 1913 using X-rays.
Rutherford continued his experiments. In 1917, he found that when he shot alpha particles into nitrogen gas, he saw signs of hydrogen nuclei. He realized that the alpha particle was knocking a hydrogen nucleus out of the nitrogen atom. This was a very important discovery! It showed that the hydrogen nucleus was a basic part of other atomic nuclei. This event is often called the discovery of the proton.
Rutherford knew hydrogen was the simplest and lightest element. He thought that hydrogen's nucleus must be a fundamental building block. So, he decided to give this new basic particle a special name. In 1920, he named it the proton. This name comes from a Greek word meaning "first." He also remembered Prout's old word "protyle." The name "proton" was first used in scientific writings in 1920.
Related pages
See also
 In Spanish: Protón para niños
In Spanish: Protón para niños
Images for kids
-
A proton detected in a cloud chamber.