Giant tube worms facts for kids
Riftia pachyptila, often called the giant tube worm, is a fascinating marine animal. It's an invertebrate, meaning it doesn't have a backbone, and it belongs to the Annelida group, which includes worms. These amazing worms live deep down on the floor of the Pacific Ocean, especially near hydrothermal vents. These vents are like underwater hot springs that release super-hot water.
Giant tube worms can handle very warm temperatures, from 2 to 30 °C, and they can even live with high levels of a chemical called hydrogen sulfide. They can grow incredibly long, up to 3 m (9 ft 10 in) (about 10 feet!), and their bodies are about 4 cm (1.6 in) (about 1.5 inches) wide.
It's important to know that the name "giant tube worm" is also sometimes used for a type of shipworm called Kuphus polythalamius. But that one is actually a bivalve mollusc (like a clam), not a worm like Riftia pachyptila.
Quick facts for kids Giant tube worms |
|
|---|---|
 |
|
| Scientific classification |
|
| Kingdom: | Animalia |
| Phylum: | Annelida |
| Class: | Polychaeta |
| Family: | Siboglinidae |
| Genus: | Riftia M. L. Jones, 1981 |
| Species: |
R. pachyptila
|
| Binomial name | |
| Riftia pachyptila M. L. Jones, 1981
|
|
| Script error: The function "autoWithCaption" does not exist. | |
Script error: No such module "Check for conflicting parameters".
Contents
Discovery
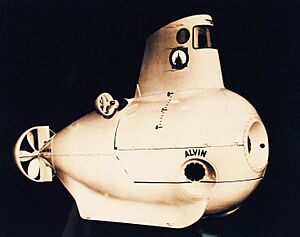
The giant tube worm was first discovered in 1977. This happened during an expedition to the Galápagos Rift using a special submarine called DSV Alvin. A geologist named Jack Corliss led the trip.
The discovery was a big surprise! The team was mainly looking at the hot vents themselves, and there weren't any biologists on board. Many of the creatures they found living near these vents had never been seen before.
Scientists already knew about hot springs near the middle of the ocean ridges. Further research showed that amazing sea life could thrive there, even though the water coming out of the vents was extremely hot (around 350–380 °C). During this trip, many samples were collected, including clams, crabs, and, for the very first time, R. pachyptila.
Life Cycle and Development
Giant tube worms start their lives as tiny, free-swimming larvae. These larvae don't have the special bacteria that adult worms need to survive. As they grow, they settle down and become attached to a surface.
Once they are settled, they get their important symbiotic bacteria from the water around them. These bacteria are like tiny helpers that live inside the worm. Young worms used to have a simple digestive system with a mouth, but this disappears as they grow into adults. The part of their body that holds the bacteria grows very large instead.
Body Structure
The giant tube worm's body is protected by a tough, white, chitin tube. Inside this tube, the worm has several unique parts.
The first part is the branchial plume. This part is bright red because it's full of blood that contains a special kind of hemoglobin. This hemoglobin is amazing because it can carry oxygen even when there's a lot of sulfide around, which usually harms hemoglobin in other animals. The plume helps the worm get important nutrients for the bacteria living inside it. If the worm feels danger, it can quickly pull its plume back into the tube and close it off with a special cap called an obturaculum.
The second part is the vestimentum. This section has strong muscles and looks a bit like wings. It also contains the worm's reproductive organs.
The middle part of the worm's body is called the trunk. This is where the trophosome is located. The trophosome is a spongy tissue packed with billions of tiny, helpful bacteria. These bacteria are called "thioautotrophic" because they make their own food using chemicals. Since adult giant tube worms don't have a mouth or a digestive system, they rely completely on these bacteria for food. This process is called chemosynthesis.
The special hemoglobin in the worm's plume can pick up both O2 and H2S from the water. These are then carried by tiny blood vessels to the bacteria in the trophosome. The bacteria use these chemicals to make food, which the worm then absorbs into its bloodstream.
The worm's blood is also very good at collecting nitrate, a chemical that can be toxic but is needed by the bacteria. The bacteria turn nitrate into ammonium, which they use to make important building blocks like amino acids. These are then shared with the worm.
The last part of the worm's body is the opistosome. This part helps anchor the worm to its tube and stores waste products from the bacteria's activities.
Symbiosis: Living Together
The discovery of giant tube worms showed scientists a new way that life can thrive without sunlight. Instead of photosynthesis (which plants use), these worms and their bacteria use chemosynthesis. This is a special partnership, or symbiosis, where the worm and the bacteria help each other.
The bacteria living inside R. pachyptila are sulfur-oxidizing bacteria. This means they get energy by breaking down sulfur compounds. Since the adult worm doesn't have a digestive system, it depends entirely on these bacteria for its food.
The worm takes in dissolved chemicals like sulfide, carbon dioxide, oxygen, and nitrogen through its plume. It then transports these chemicals through its blood system to the trophosome, where the bacteria live. The bacteria are held inside special cells within the worm, so they don't touch the outside water directly. This means they rely on the worm to get all the nutrients they need and to get rid of their waste. In return, the bacteria create food for the worm, helping it grow and survive.
Scientists first realized this partnership was happening by looking at the worms under a microscope and studying their chemicals. They found that the bacteria in the trophosome use sulfur compounds to make energy (ATP) and then use that energy to create food from carbon dioxide. The worm helps by bringing the necessary chemicals (sulfide, oxygen, and carbon dioxide) to the bacteria, and the bacteria give the worm the food it needs.
Special Bacteria Helpers
Many different types of bacteria live with R. pachyptila. Some belong to a group called Campylobacterota, and others are from groups like Delta-, Alpha-, and Gammaproteobacteria. One interesting bacterium, Candidatus Endoriftia persephone, can use different ways to make food depending on what's available. It can even live freely in the environment or inside the worm.
These bacteria get energy by breaking down sulfur compounds like hydrogen sulfide. This energy is used to make ATP, which powers their food-making process (the Calvin cycle). The worm provides the bacteria with the chemicals they need, and in return, the bacteria give the worm organic matter (food). Because the worm has no mouth, it completely depends on these bacteria to live.
Here's how it works: 1. Sulfide from the water enters the worm's blood. 2. The worm's hemoglobin binds to both sulfide and oxygen, carrying them to the trophosome where the bacteria are. 3. Inside the trophosome, the bacteria use the sulfide and oxygen to create energy. This energy is then used to turn carbon dioxide into food for both themselves and the worm.
To do all this, the giant tube worm needs to get both oxygen (from oxygen-rich water) and sulfide (from the vent fluids). It can do this because its special hemoglobin can bind to sulfide without being harmed, and then transport it safely to the bacteria.
Carbon Fixation and Food
Unlike most animals that breathe out carbon dioxide as waste, the giant tube worm and its bacteria actually need to take in carbon dioxide. This is because the bacteria use it to make their food.
Deep-sea water has a lot of carbon in the form of bicarbonate. However, the worm's blood has a higher pH, which helps it take in carbon dioxide more easily. The vent fluids also provide a lot of carbon dioxide.
Once the bacteria make food from carbon dioxide, it needs to get to the worm's body. The food is transported through the worm's blood from the trophosome to other tissues. Studies show that the worm starts getting this food very quickly after the bacteria make it.
How They Get Their Bacteria
Scientists believe that R. pachyptila gets its helpful bacteria from the environment around it. This is called environmental transfer.
Evidence for this comes from several studies:
- Different types of tube worms (Riftia, Oasisia, and Tevnia) that live in the same areas share the same kind of bacteria. This suggests the bacteria are picked up from the environment.
- When scientists looked at R. pachyptila eggs, they didn't find any of the symbiotic bacteria. This means the bacteria are not passed down from parent to offspring (which would be "vertical transfer").
- Studies also found that the bacteria have genes for making flagella, which are like tiny tails that help bacteria swim. If the bacteria were passed down from parents, they wouldn't need to swim. But if they come from the environment, having flagella would be very helpful for them to reach and enter the worm.
All this evidence points to the idea that giant tube worms get their special bacterial helpers from the water around them.
Reproduction
Male giant tube worms release their sperm into the sea water. These groups of sperm, called spermatozeugmata, can stay together for a while in the unique conditions near the hydrothermal vents. Usually, these sperm groups then swim into the female's tube to fertilize her eggs.
Growth Rate and Age
The giant tube worm has one of the fastest growth rates of any known marine invertebrate. These worms can move into a new area, grow to be able to reproduce, and reach a length of 4.9 feet (1.5 m) in less than two years!
See also
 In Spanish: Gusano de tubo gigante para niños
In Spanish: Gusano de tubo gigante para niños
- Siboglinidae
- Lamellibrachia
- List of long-living organisms
- Maximum life span
- Pompeii worm
- Chemosynthesis