Ronald Breslow facts for kids
Quick facts for kids
Ronald Breslow
|
|
|---|---|
 |
|
| Born |
Ronald Charles D. Breslow
March 14, 1931 |
| Died | October 25, 2017 (aged 86) New York City, US
|
| Alma mater | Harvard University |
| Awards | ACS Award in Pure Chemistry (1966) NAS Award in Chemical Sciences (1989) National Medal of Science (1991) Priestley Medal (1999) Othmer Gold Medal (2006) Perkin Medal (2010) AIC Gold Medal (2014) |
| Scientific career | |
| Fields | Chemistry |
| Institutions | Columbia University |
| Thesis | Studies on magnamycin (1956) |
| Doctoral advisor | Robert Burns Woodward |
| Doctoral students |
|
| Other notable students | Anthony Czarnik |
Ronald Charles David Breslow (born March 14, 1931 – died October 25, 2017) was an American chemist from Rahway, New Jersey. He was a professor at Columbia University. He worked in the Chemistry Department and also with the Biological Sciences and Pharmacology departments. He taught at Columbia starting in 1956 and used to be the head of the university's chemistry department.
Who was Ronald Breslow?
Ronald Breslow was born in Rahway, New Jersey. His parents were Gladys (Fellows) and Alexander E. Breslow. He loved creating new molecules with special features. He also enjoyed studying what these features could do.
What did Ronald Breslow discover?
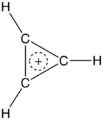
One of his big discoveries was the cyclopropenyl cation. This is the simplest aromatic system. It was the first aromatic compound made with something other than six electrons in a ring.
He also figured out how an important enzyme, thiamin diphosphate, works. This enzyme helps break down pyruvate. He used a special method called proton NMR to understand this. His work on how molecules bind to cyclodextrins also became very important. It helped shape modern organic and biological chemistry.
Breslow also helped find a medicine called SAHA (Vorinostat). This medicine is approved to treat a type of cancer called cutaneous T-cell lymphoma.
Where did Ronald Breslow study?
Ronald Breslow earned his bachelor's, master's, and Ph.D. degrees from Harvard University. His Ph.D. advisor was R. B. Woodward, a famous chemist.
Many of Breslow's students became successful scientists. One of them, Robert Grubbs, won the Nobel Prize in Chemistry in 2005. Another student, Doug La Follette, became the Secretary of State of Wisconsin.
What awards did Ronald Breslow receive?
Ronald Breslow received many important awards for his work. These include:
- The National Medal of Science in 1991.
- The Welch Award.
- The Arthur C. Cope Award in 1987.
- The NAS Award in Chemical Sciences.
- The ACS Award in Pure Chemistry in 1966.
- The Othmer Gold Medal in 2006.
- The Priestley Medal.
- The 2014 American Institute of Chemists (AIC) Gold Medal.
Columbia University also gave him awards for his great teaching skills. These were the Mark Van Doren Award and the Great Teacher Award. He was the president of the American Chemical Society (ACS) in 1996. He also led the chemistry division of the National Academy of Sciences from 1974 to 1977.
In 1997, a magazine called Chemical & Engineering News named him one of the top 75 people who helped chemistry in the past 75 years. The American Chemical Society (ACS) even named an award after him: the Ronald Breslow Award for Achievement in Biomimetic Chemistry. This award is given out every year.
What groups was Ronald Breslow a member of?
Ronald Breslow was a member of many important scientific groups. These included:
- The National Academy of Sciences.
- The American Academy of Arts and Sciences.
- The European Academy of Sciences.
- The American Philosophical Society.
He was also a foreign member of the Royal Society in the UK. He was an honorary member of many other science groups around the world.
How did life's building blocks become "handed"?
The basic building blocks of life, like amino acids and sugars, can exist in two forms. These forms are like your left and right hands; they are mirror images of each other. Scientists call them L (left) and D (right).
However, life on Earth uses mostly L-amino acids and D-sugars. If living things had a mix of both L and D forms, important molecules like proteins and DNA would not work correctly. Scientists have wondered for a long time why life chose one "hand" over the other.
Did life's "handedness" come from space?
There is a lot of evidence that this "handedness," or chirality, might have come from outer space. Scientists found special amino acids called α-methyl amino acids inside the Murchison meteorite. These amino acids had a slightly higher amount of the L form.
These α-methyl amino acids are thought to be from space because they have a lot of a heavier carbon (13C) and deuterium. Also, these types of amino acids are usually not found in chemistry on Earth.
Some people worried that these amino acids would burn up when the meteorite crashed into Earth. But the amino acids were found inside the meteorite, which acted like a protective shield. Unlike regular amino acids, α-methyl amino acids do not change their "handedness" easily over long periods of time.
How did the L form become more common?
Scientists are still debating how the L form of α-methyl amino acids became more common. One popular idea is that a special kind of light in space, called right circularly polarized light, destroyed more of the D form.
This type of light is produced by objects like synchrotrons. Neutron stars could act like synchrotrons, sending right-polarized light towards our part of the universe. However, some astronomers say this light only happens in the infrared range. This energy is too low to destroy molecules.
Another challenge is figuring out how a small excess of L-α-methyl amino acids could lead to a large amount of L-regular amino acids.
Researchers found that if only L-α-methyl amino acids were present, the D form of the new amino acid was slightly favored.
However, when scientists added copper to the reaction, the L form was produced. Meteors have been found to contain both copper and zinc, which supports using these metals in experiments. When zinc was used, the L form was not preferred. Calculations suggest that copper forms a special shape that helps create the L-amino acid.
How can a small difference become a big difference?
If there is a small excess of one "handed" form, it can be made much larger. This is done by controlling how easily the pure and mixed crystals dissolve. When both L and D forms are present, they often form a mixed crystal structure. This mixed crystal is usually more stable and less soluble than the pure L or D crystal.
So, if you have a small excess of one form, you can evaporate the liquid. The mixed form will then fall out of the solution first. This leaves more of the pure form behind. Researchers have shown that they can start with just 1% more of the L form and end up with a solution that is 95% L and 5% D.
These findings, especially the idea about light from space, led Breslow to suggest that life could exist in other parts of the universe with D-amino acids and L-sugars.
Images for kids
 | Roy Wilkins |
 | John Lewis |
 | Linda Carol Brown |