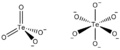Tellurate facts for kids
Tellurate is a special kind of ion. Think of an ion as a tiny particle that has an electric charge. Tellurate ions are made from the element tellurium and oxygen. They can have different chemical formulas, like TeO42- or TeO66-. When these tellurate ions combine with other elements, they form chemical compounds called tellurates.
What Tellurates Do
Tellurates are known as strong oxidizing agents. This means they can easily take electrons from other substances during a chemical reaction. They are quite similar to another group of chemicals called selenates. When tellurates mix with acids, they create a substance known as telluric acid.
How Tellurates Are Made
One common example is sodium tellurate, which has the formula Na2TeO4. You can make sodium tellurate in a few ways. One way is to react telluric acid with sodium hydroxide. Another way is to use a process called oxidation on a chemical called sodium tellurite. Sodium tellurate is very similar to sodium selenate.
Related pages
Images for kids
See also
 In Spanish: Telurato para niños
In Spanish: Telurato para niños