Telluric acid facts for kids
Quick facts for kids Telluric acid |
|
|---|---|
 |
|
 |
|
| IUPAC name | Hexahydroxidotellurium |
| Other names | Orthotelluric acid |
| Identifiers | |
| CAS number | |
| PubChem | |
| ChEBI | CHEBI:30463 |
| SMILES | O[Te](O)(O)(O)(O)O |
|
InChI
InChI=1/H2O4Te/c1-5(2,3)4/h(H2,1,2,3,4)
|
|
| Properties | |
| Molecular formula | |
| Molar mass | 0 g mol-1 |
| Appearance | White monoclinic crystals |
| Density | 3.07 g/cm3 |
| Melting point | |
| 50.1 g/100 ml at 30°C | |
| Acidity (pKa) | 7.68, 11.0 at 18°C |
| Structure | |
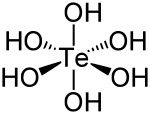
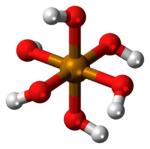
| Molecular shape | octahedral |
| Dipole moment | 0 D |
| Hazards | |
| Main hazards | corrosive |
| Related compounds | |
| Other anions | hydrotelluric acid tellurous acid hydrogen telluride |
| Related compounds | Teflic acid, Sulfuric acid Selenic acid |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Telluric acid is a special kind of chemical compound. It is an acid, which means it can react with other chemicals. Its chemical formula is H6TeO6. This formula tells us it has hydrogen, oxygen, and an element called tellurium.
Telluric acid is known as a weak acid. This means it does not react as strongly as some other acids. It can react with strong bases to create new compounds called tellurates. Telluric acid is also a powerful oxidizing agent. This means it can cause other chemicals to lose electrons. It is important to know that telluric acid can be harmful if it touches your skin or eyes. It is a corrosive substance.
How Telluric Acid Is Made
Scientists can make telluric acid in a lab. One way is to mix tellurium dioxide with chromium trioxide. Another way is to react tellurium dioxide with hydrogen peroxide. These reactions help create the telluric acid we use.
What Telluric Acid Is Used For
Telluric acid is mainly used to make other tellurium compounds. For example, it helps create sodium tellurate. These other compounds can then be used for different purposes in chemistry.
Related pages
See also
 In Spanish: Ácido telúrico para niños
In Spanish: Ácido telúrico para niños
 | Audre Lorde |
 | John Berry Meachum |
 | Ferdinand Lee Barnett |

