Tellurous acid facts for kids
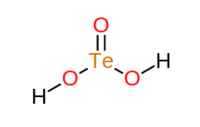
Tellurous acid is a special kind of chemical compound that is also an acid. Its chemical formula is H2TeO3. This means it is made up of hydrogen atoms, oxygen atoms, and a tellurium atom. It contains hydrogen and tellurite ions.
You can make tellurous acid by mixing tellurium dioxide with water. However, it's not as easy to make as another acid called telluric acid.
Tellurous acid is known as a weak acid. This means it doesn't break apart completely into ions when it's in water, unlike strong acids. It can react with bases to create new compounds called tellurites.
It is also a weak oxidizing agent and a weak reducing agent. This means it can sometimes cause other chemicals to lose electrons (oxidize them) or gain electrons (reduce them), but it's not very strong at doing either. Tellurous acid can easily change back into tellurium dioxide and water.
Quick facts for kids Tellurous acid |
|
|---|---|
 |
|
| IUPAC name | Tellurous acid |
| Other names | Tellurium dioxide hydrate, tellurium(IV) oxide hydrate |
| Identifiers | |
| CAS number | |
| PubChem | |
| ChEBI | CHEBI:30465 |
| SMILES | O=[Te](O)O |
|
InChI
InChI=1/H2O3Te/c1-4(2)3/h(H2,1,2,3)
|
|
| Properties | |
| Molecular formula | |
| Molar mass | 0 g mol-1 |
| Appearance | colorless crystals |
| Density | ~ 3 g/cm3 |
| Boiling point | |
| negligible | |
| Acidity (pKa) | pKa(1) = 2.48, pKa(2) = 7.70 |
| Structure | |
| Crystal structure | unknown |
| Molecular shape | pyramidal at Te |
| Related compounds | |
| Other anions | Selenous acid Sulfurous acid |
| Other cations | Sodium tellurite |
| Related compounds | Telluric acid Selenic acid Sulfuric acid |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Related pages
See also
 In Spanish: Ácido teluroso para niños
In Spanish: Ácido teluroso para niños
 | May Edward Chinn |
 | Rebecca Cole |
 | Alexa Canady |
 | Dorothy Lavinia Brown |

