Thermodynamic entropy facts for kids
Thermodynamic entropy is a way to measure how spread out or "disorganized" energy is within a group of atoms or molecules. It tells us how much energy is available to do work. We measure it in joules of energy per unit kelvin. Entropy is a key idea in the laws of thermodynamics.
Imagine you have ten units of energy. If this energy is perfectly organized, you can use all ten units to do work. But if the energy becomes less organized, meaning its entropy increases, you might only be able to use six units of work, even though you still have ten units of energy in total. This is because some energy has spread out and is no longer useful for work.
Contents
What Happens When Energy Spreads Out?
When energy has spread out as much as it can, we say it has reached "total entropy." At this point, there is no more useful energy left to spend.
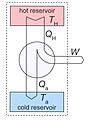
Think about a cup of hot tea. The tea has a lot of heat energy compared to the room around it. Over time, the heat from the tea will naturally spread into the room. The tea will get colder. This happens because the heat energy moves from the warm tea to the cooler air. Once the tea becomes cold, there is no more heat that can easily spread out from it. The tea and the room have reached a state of total entropy together.
Open and Closed Systems
To understand how energy moves, we can think of two types of "rooms" or systems: an open system and a closed system.
- An open system means that energy, like heat, can freely move in and out of the "room."
- A closed system means the "room" is sealed off from the outside. No energy can enter or leave it.
In our tea example, if the room was a closed system, no new energy could come in. The tea would just get cold and stay that way. But we can change this!
How Energy Can Be Reused
If we put a heater into the room, we make it an open system. When we turn on the heater, new energy comes into the room. This new heat can warm up the cup of tea again. When new energy is added, the entropy of the tea system decreases. The heat from the heater goes into the tea, and then it can spread out into the room again. This process continues until total entropy is reached once more. This idea is a big part of the second law of thermodynamics.
A great real-life example of an open system is the Earth. Our planet gets a huge amount of energy from the Sun every single day. This constant energy allows plants to grow and keeps water liquid. If the Sun's energy were taken away, plants would die, and water would freeze. This is because the Earth's surface would become too cold without that incoming energy.
Images for kids