Thial facts for kids
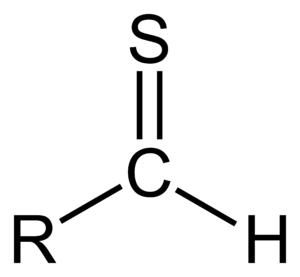
A thial, also known as a thioaldehyde, is a special type of molecule. It has a specific group of atoms called a functional group. This group is made of a carbon atom, a sulfur atom, and a hydrogen atom, all connected in a particular way.
Contents
What is a Thial?
Thials are very similar to another type of molecule called an aldehyde. The main difference is that in a thial, the oxygen atom found in an aldehyde is replaced by a sulfur atom. This sulfur atom is connected to the carbon atom with a double bond. It also has two pairs of electrons that are not shared, called lone pairs.
Thials vs. Aldehydes
Imagine an aldehyde as a car with an oxygen engine. A thial is like the same car, but its oxygen engine has been swapped out for a sulfur engine. This small change makes a big difference in how the molecule behaves.
Why are Thials Reactive?
Thials are known for being very reactive. This means they like to quickly join with other molecules. They are very good at attracting electrons from other molecules. Because they react so easily, it's hard for scientists to make them and keep them stable. As soon as they form, they often react with themselves or other nearby molecules. They can take part in very fast chemical reactions, like a Diels-Alder reaction. However, if the thial has very large or "bulky" groups of atoms attached to it, these groups can protect the reactive part, making the thial a bit more stable.
What are Thioketones?
If the hydrogen atom in a thial is replaced with another group of atoms, the molecule changes its name to a thioketone. Thioketones are generally more stable than thials. This means they don't react as quickly and are easier to study and use.
See also
 In Spanish: Tial para niños
In Spanish: Tial para niños