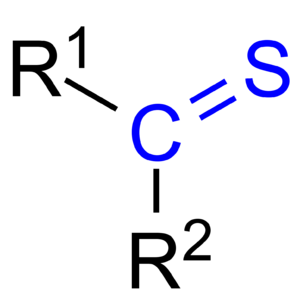Thioketone facts for kids
A thioketone (also called a thione) is a special type of molecule. It has a group of atoms called R2C=S. Think of it like a regular ketone, but with a twist! In a ketone, you have an oxygen atom. In a thioketone, that oxygen atom is replaced by a sulfur atom.
This sulfur atom is connected to a carbon atom with a double bond. It also has two special pairs of electrons called lone pairs. These lone pairs are important for how the molecule behaves.
What Makes Thioketones Special?
Thioketones are known for being very reactive. This means they like to react with other molecules easily. They are good at attracting electrons from other substances. Because of this, many thioketones are not very stable on their own.
They often try to form ring shapes. They do this through reactions called cycloadditions. One famous example of a similar reaction is the Diels–Alder reaction.
Thioketones vs. Thials
Sometimes, one of the 'R' groups in a thioketone can be a hydrogen atom. When this happens, the molecule becomes the sulfur version of an aldehyde. This type of molecule is called a thial. Thials are even more reactive than thioketones! They are very eager to react and change into other forms.
Images for kids
See also
 In Spanish: Tiocetona para niños
In Spanish: Tiocetona para niños