Calcium nitride facts for kids
Quick facts for kids Calcium nitride |
|
|---|---|
 |
|
| IUPAC name | Calcium nitride |
| Other names | tricalcium dinitride |
| Identifiers | |
| CAS number | |
| PubChem | |
| EC number | 234-592-9 |
| Properties | |
| Molecular formula | |
| Molar mass | 0 g mol-1 |
| Appearance | red-brown crystalline solid |
| Density | 2.670 g/cm3 2.63 g/cm3 (17 °C) |
| Melting point | |
| decomposes | |
| Structure | |
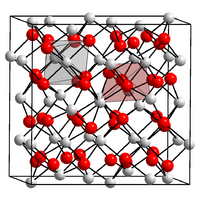
| Crystal structure | Cubic, cI80 |
| Space group | Ia-3, No. 206 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Calcium nitride is a chemical compound. It is made from the elements calcium and nitrogen. Its chemical formula is Ca3N2. This means that each molecule of calcium nitride contains three atoms of calcium and two atoms of nitrogen.
Contents
What is Calcium Nitride?
Calcium nitride is a solid substance. It has a distinct red-brown color. It is also a crystalline solid, meaning its atoms are arranged in a very organized pattern.
How Calcium Nitride Reacts
Calcium nitride reacts strongly with water. When it touches water, it breaks down. This reaction creates two new substances: calcium hydroxide and ammonia. Ammonia is a gas with a strong smell.
It also reacts with hydrogen gas. This reaction forms calcium hydride and calcium amide. These are other chemical compounds.
How is Calcium Nitride Made?
Calcium nitride can be made in a couple of ways.
Making it with Nitrogen
One way to make calcium nitride is by burning calcium metal. This is done in an atmosphere of pure nitrogen gas. The calcium and nitrogen combine to form the new compound.
Making it from Air
Calcium nitride can also form when calcium burns in regular air. Air contains about 78% nitrogen. So, when calcium burns in air, it reacts with the nitrogen present. This reaction also produces calcium nitride.
What is Calcium Nitride Used For?
Calcium nitride is useful in chemistry. It can be used as a source of the reactive nitride ion. A nitride ion is a nitrogen atom with an extra electric charge. This makes it very reactive in chemical processes.
Related pages
See also
 In Spanish: Nitruro de calcio para niños
In Spanish: Nitruro de calcio para niños
 | Dorothy Vaughan |
 | Charles Henry Turner |
 | Hildrus Poindexter |
 | Henry Cecil McBay |

