Diffusion facts for kids
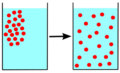
Diffusion is a cool way that tiny particles, called molecules, move around. Imagine a crowded room where everyone wants more space. Molecules do something similar! They naturally spread out from areas where there are lots of them (high concentration) to areas where there are fewer (low concentration).
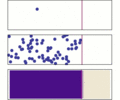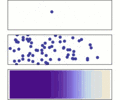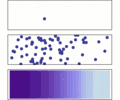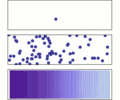
This spreading out usually happens in gases or liquids. You can easily see diffusion in action when you mix two liquids in a clear container. It's all about the constant, random movement of these particles. They bump into each other and keep moving until they are spread out evenly. Diffusion only works with gases and liquids.
Contents
Everyday Examples of Diffusion
Here are some everyday examples of diffusion:
- When you put a sugar cube in a glass of water, the sugar molecules slowly spread throughout the water.
- If someone opens a bottle of perfume, its smell quickly travels across the room.
- The smell of ammonia from a cleaning product can spread from the front to the back of a classroom.
- A drop of food coloring in a beaker of water will spread out and color the whole liquid.
How Molecules Move
Molecules tend to move from places where there are many of them to places where there are fewer. They do this just by moving randomly. Think of it like this: if you have a lot of people in one corner of a room, they will naturally spread out until the room is more evenly filled.
This process is very important in your body! For example, there is more oxygen in your lungs than in your blood. So, oxygen molecules will naturally move from your lungs into your blood. At the same time, there is more carbon dioxide in your blood than in your lungs. So, carbon dioxide molecules will move from your blood into your lungs to be breathed out.
Diffusion in Cells
Diffusion also happens inside your body's tiny cells. Small molecules can easily diffuse, or pass through, the cell's outer layer called the cell membrane. However, larger molecules need special help to get through. They often need the cell to use energy to move them across. This special process is called active transport.
Areas where molecules are packed closely together will naturally spread out. This happens because the molecules are always moving and bumping into each other. They will keep spreading until they can't spread any further, like when they hit the edge of a container.
See also
 In Spanish: Difusión (física) para niños
In Spanish: Difusión (física) para niños
Images for kids
 | Frances Mary Albrier |
 | Whitney Young |
 | Muhammad Ali |