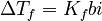Freezing-point depression facts for kids
Freezing-point depression is when a liquid's freezing temperature drops because you've added another substance to it. Think of it like adding salt to water. Pure water freezes at 0 degrees Celsius (32 degrees Fahrenheit). But if you add salt, the water needs to get even colder before it turns into ice!
This happens because the added substance, called the solute, gets in the way of the liquid's own molecules, called the solvent, trying to freeze. It makes it harder for them to line up and form a solid structure. So, the mixture has to reach a lower temperature to freeze.
You see freezing-point depression in many places:
- Adding salt to roads to melt ice in winter.
- Using special liquids (antifreeze) in car engines to stop them from freezing.
- How ice cream stays soft and scoopable even when it's below 0 °C.
- How some animals and plants survive in very cold places without freezing solid.
Contents
What Makes the Freezing Point Drop?
When a pure liquid freezes, its molecules slow down and arrange themselves into a neat, solid pattern. Imagine building a wall with identical bricks – it's easy to make a perfect pattern.
How Solutes Change Freezing
Now, imagine trying to build that same wall, but someone keeps throwing different-shaped stones into your pile of bricks. It becomes much harder to make a neat, organized wall! The stones (solute) disrupt the bricks (solvent) from forming their solid structure.
In the same way, when you add a solute to a liquid, the solute particles spread out among the solvent particles. This makes the mixture more "disordered" or random. For the solvent particles to freeze and become organized, they need to get much colder to overcome this extra disorder caused by the solute. This is why the freezing point drops.
It's not that the solute stops the solvent from freezing completely. It just makes it less likely for the solvent molecules to find each other and lock into place at the usual freezing temperature. A lower temperature is needed to force them into that solid arrangement.
Everyday Uses of Freezing-Point Depression
This scientific idea has many helpful uses in our daily lives and in nature!
Keeping Roads Safe in Winter
One of the most common uses is putting salt on icy roads and sidewalks. When salt (like sodium chloride) mixes with the ice, it lowers the freezing point of the water. This means the ice can melt even when the temperature is a few degrees below 0 °C. This helps prevent dangerous, slippery conditions.
If it gets extremely cold, below about -21 °C (-6 °F), regular salt might not work as well. In those cases, other salts like calcium chloride or magnesium chloride might be used because they can lower the freezing point even more. Airports sometimes use different, less corrosive chemicals like sodium formate to protect aircraft and equipment.
Protecting Car Engines
Car radiators contain a mixture of water and a special liquid called antifreeze (often ethylene glycol). This mixture uses freezing-point depression to stop the water in the engine from freezing solid in cold weather. If the water froze, it could expand and damage the engine! Antifreeze also helps prevent the water from boiling in hot weather, which is another useful property.
Nature's Antifreeze: Surviving the Cold
Some amazing animals and plants have developed their own ways to use freezing-point depression to survive in very cold environments. They produce special compounds inside their bodies, like glycerol or sorbitol. These compounds act like natural antifreeze, lowering the freezing point of the water in their cells. This stops them from freezing solid, even when the world around them is icy cold.
For example, some arctic fish, like the rainbow smelt, make glycerol to live in frozen waters during winter. The spring peeper frog (Pseudacris crucifer) can even increase the amount of glucose (a type of sugar) in its blood when temperatures drop. This acts as an antifreeze, protecting its organs from freezing.
Science in the Lab and Industry
Scientists and industries also use freezing-point depression.
- It can help check how pure a substance is. If a substance has impurities, its freezing point will be lower than expected.
- The dairy industry uses it to make sure milk hasn't been watered down. Pure milk has a specific freezing point, and if it's higher, it means extra water has been added.
How We Measure Freezing-Point Drop
Scientists have a way to calculate how much the freezing point will drop when a solute is added, especially for solutions that aren't too concentrated.
The formula looks like this:
Let's break down what each part means:
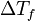 (pronounced "delta T eff") is the amount the freezing point drops. It's the difference between the freezing point of the pure liquid and the freezing point of the mixture.
(pronounced "delta T eff") is the amount the freezing point drops. It's the difference between the freezing point of the pure liquid and the freezing point of the mixture.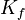 is called the cryoscopic constant. This is a special number that depends only on the pure liquid (the solvent). Every liquid has its own
is called the cryoscopic constant. This is a special number that depends only on the pure liquid (the solvent). Every liquid has its own 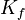 value. A higher
value. A higher 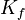 means the freezing point will drop more for the same amount of solute.
means the freezing point will drop more for the same amount of solute.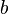 is the molality. This tells us how much solute is dissolved in a certain amount of solvent. It's measured in moles of solute per kilogram of solvent.
is the molality. This tells us how much solute is dissolved in a certain amount of solvent. It's measured in moles of solute per kilogram of solvent.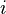 is the van 't Hoff factor. This number tells us how many particles a solute breaks into when it dissolves. For example, if you dissolve table salt (NaCl) in water, it breaks into two particles (Na+ and Cl-), so i would be 2. If a substance doesn't break apart, i is 1.
is the van 't Hoff factor. This number tells us how many particles a solute breaks into when it dissolves. For example, if you dissolve table salt (NaCl) in water, it breaks into two particles (Na+ and Cl-), so i would be 2. If a substance doesn't break apart, i is 1.
Here are some 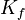 values for common liquids:
values for common liquids:
| Compound | Freezing point (°C) | Kf in K⋅kg/mol |
|---|---|---|
| Acetic acid | 16.6 | 3.90 |
| Benzene | 5.5 | 5.12 |
| Camphor | 179.8 | 39.7 |
| Water | 0 | 1.86 |
Notice how water has a 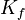 of 1.86. This means for every mole of solute particles added to a kilogram of water, the freezing point drops by 1.86 °C.
of 1.86. This means for every mole of solute particles added to a kilogram of water, the freezing point drops by 1.86 °C.
Ethanol and Water Mixtures
This graph shows how the freezing point changes when you mix ethanol (alcohol) with water. As you add more ethanol, the freezing point of the mixture gets lower and lower. This is why alcoholic drinks don't freeze as easily as pure water!
See also
- Melting-point depression
- Boiling-point elevation
- Colligative properties
- Deicing
- Eutectic point
- Frigorific mixture
- List of boiling and freezing information of solvents
- Snow removal
 | Emma Amos |
 | Edward Mitchell Bannister |
 | Larry D. Alexander |
 | Ernie Barnes |