Homologous series facts for kids
In chemistry, a homologous series is like a family of chemical compounds. All members of this family share a similar general formula. This means their basic chemical recipe is the same. The compounds in the series usually differ by a simple, repeating part. Think of it like adding one LEGO brick at a time to a chain.
These compounds also have similar functional groups. A functional group is a special group of atoms that gives a molecule its main chemical behaviors. Because they have similar functional groups, compounds in a homologous series have similar chemical and physical properties. For example, they might react in similar ways. Their properties change slowly as you go along the series. This is because the molecules get a little bigger and heavier.
Contents
What is a Homologous Series?
A homologous series is a group of chemical compounds that are very much alike. They all follow the same basic chemical pattern. Each member in the series is slightly different from the one before it. This difference is usually a simple repeating unit, like a carbon atom with two hydrogen atoms attached (a -CH₂- group).
How Compounds Are Related
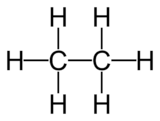
Imagine you have a train. Each car on the train is similar, but the train can get longer by adding more cars. In a homologous series, the "cars" are often -CH₂- groups. So, each compound in the series has one more -CH₂- group than the one before it.
Examples of Homologous Series
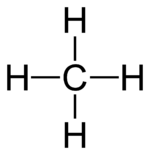
There are many examples of these chemical families. One common example is the alkanes. These are simple organic compounds made only of carbon and hydrogen.
Each one adds another -CH₂- unit.
Other examples include:
- Alcohols: These compounds have an -OH (hydroxyl) group.
- Aldehydes: These have a -CHO group.
- Carboxylic acids: These have a -COOH group.
All these groups give the compounds their special properties.
Properties That Change Gradually
When you look at a homologous series, you'll notice that their physical properties change in a smooth way. For example, as the molecules get bigger:
- Their boiling points usually go up.
- Their melting points also tend to increase.
- Their density might also change gradually.
This happens because larger molecules have more atoms. This means they are heavier and have stronger forces between them. It takes more energy to break these forces, so they need higher temperatures to melt or boil.
Images for kids