Iron(II) chloride facts for kids
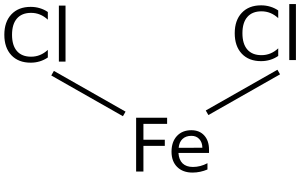
Iron(II) chloride, also called ferrous chloride, is a chemical compound. Think of it as a tiny building block made from two different parts: iron and chloride ions. In this compound, the iron atom has a special electrical charge, which chemists call an "oxidation state" of +2.
Contents
What is Iron(II) Chloride Like?
Iron(II) chloride can look different depending on if it has water mixed in. Without water, it is a grayish-white solid. When it has water molecules attached, it looks greenish.
If it touches oxygen from the air, it can change. It turns into a mix of iron(III) chloride and iron(III) oxide, which are brownish-yellow. This process is called oxidation.
When you put iron(II) chloride in water, it dissolves. It forms a yellowish-green liquid. This compound is also known as a reducing agent. This means it can give away electrons to other chemicals in a reaction.
It also reacts with bases. When this happens, it forms a greenish substance called iron(II) hydroxide.
How is Iron(II) Chloride Made?
One way to make iron(II) chloride is to dissolve iron metal in hydrochloric acid. Imagine putting iron pieces into a strong acid; they will react and form this compound.
You can also make it by mixing iron with iron(III) chloride. In this reaction, the iron helps to change the iron(III) chloride into iron(II) chloride. Other chemicals that are good at giving away electrons can also turn iron(III) chloride into iron(II) chloride.
What is Iron(II) Chloride Used For?
Iron(II) chloride is not as commonly used as some other iron compounds. For example, iron(II) sulfate and iron(III) chloride are used more often.
However, it has some important jobs. It is used to help clean wastewater. It helps to remove harmful substances from the water.
It can also be used in organic chemistry. Here, it acts as a reducing agent. This means it helps certain chemical reactions happen by giving electrons to other molecules.
Related Pages
Images for kids
See also
 In Spanish: Cloruro de hierro(II) para niños
In Spanish: Cloruro de hierro(II) para niños
 | Jackie Robinson |
 | Jack Johnson |
 | Althea Gibson |
 | Arthur Ashe |
 | Muhammad Ali |