Jaqueline Kiplinger facts for kids
Quick facts for kids
Jaqueline Kiplinger
|
|
|---|---|
 |
|
| Nationality | American |
| Alma mater | University of Colorado University of Utah |
| Scientific career | |
| Institutions | Los Alamos National Laboratory |
| Thesis | Activation and Functionalization of Carbon-Fluorine Bonds by Transition Metal Complexes (1996) |
Jaqueline Kiplinger is an American chemist who studies special types of chemicals. She focuses on organometallic actinide chemistry. Actinides are a group of elements like uranium and thorium.
Throughout her career, she has worked a lot with chemicals containing fluorine (called fluorocarbons) and with actinides. Currently, she is a top scientist at Los Alamos National Laboratory (LANL). Her work there helps develop chemistry for the United States’ national defense and energy needs.
Education and Early Research
Kiplinger earned her bachelor's degree in chemistry in 1990. She studied at the University of Colorado at Colorado Springs. While there, she published several papers with her professor, Ruminiski. These papers were about how iron and ruthenium chemicals connect with other molecules.
She then went to the University of Utah for her graduate studies. She worked with Professor Thomas Richmond. Her research focused on breaking strong chemical bonds between carbon and fluorine atoms. These bonds are usually very hard to break. This is because fluorine is very "greedy" for electrons, and the bond itself is very strong.
One important discovery she made was changing a carbon-fluorine bond into a carbon-carbon bond. She used a metal called tungsten to do this. She also found ways to use zirconium to help break carbon-fluorine bonds in a process called hydrogenolysis.
From 1996 to 1999, Kiplinger continued her research at UC Berkeley. She worked as a postdoctoral researcher with Professor Robert G. Bergman.
Career at Los Alamos
After her postdoctoral work, Kiplinger received a special fellowship at LANL. There, she began a new research program. This program focused on the chemistry of actinides and lanthanides. Lanthanides are another group of elements similar to actinides.
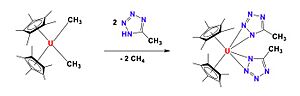
In 2002, Kiplinger and her team made an important discovery. They created the first f-block element complex that contained a ketimido group. This was the first of many papers they published about uranium chemistry. Soon after, they also created the first actinide hydrazonato complex using uranium. During this time, Kiplinger also found better ways to make certain uranium-based compounds.
Kiplinger continued her research at LANL after her fellowship ended. She is still a leading scientist in the chemistry division there today. She kept developing new chemistry for lanthanides and actinides. In 2013, she published how to make the first uranium and thorium halide complexes with special ligands.
In 2016, Kiplinger announced the creation of a unique thorium diazide coordination polymer. This was the first time such a polymer involving an actinide had been made. A polymer is a large molecule made of many repeating smaller units. This thorium polymer is special because it can grow infinitely. Other similar actinide compounds, like those with uranium, only form smaller groups of three.
By changing parts of the molecules, different types of these polymers can be formed. Her recent work has focused on uranium and thorium compounds that have many nitrogen atoms. For example, her team found that certain parts of these compounds act as "sigma donors."
Another important part of her research involves redox chemistry with uranium hydrides. Redox chemistry is about how electrons are transferred between molecules. She showed that a chemical called phenylsilane can be used to create different forms of uranium. These forms include uranium (III), uranium (IV), and uranium (VI) complexes.
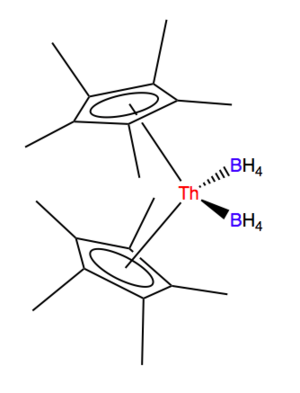
Her more recent work describes a new way to make uranium and thorium metallocene borohydrides. The uranium complex had been made before. However, her new method uses different starting materials. This new way results in more of the product and can be done in milder conditions. While uranium borohydride was first made in the 1940s, her work created the first example of a thorium metallocene borohydride.
Awards and Recognition
- 2018: Fellow of the American Chemical Society
- 2017: IUPAC International Distinguished Women in Chemistry Award
- 2016: University of Utah Distinguished Chemistry Alumna Award
- 2015: ACS F. Albert Cotton Award in Synthetic Inorganic Chemistry
- 2013-2014: Organometallic Subdivision Chair, ACS Division of Inorganic Chemistry
- 2012: R&D 100 Award for U-TURN: Turning Uranium Around Process
- 2012: Fellow of the Royal Society of Chemistry
- 2012: National Nuclear Security Administration Environmental Stewardship Award
- 2010: National Nuclear Security Administration Best-in-Class Pollution Prevention Award
- 1998: ACS Nobel Laureate Signature Award for the best Ph.D. thesis in the U.S.
 | James B. Knighten |
 | Azellia White |
 | Willa Brown |