Marburg virus facts for kids
Quick facts for kids Marburg virus |
|
|---|---|
 |
|
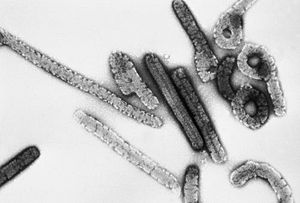
| Transmission electron micrograph of Marburg virus | |
| Virus classification |
|
| (unranked): | Virus |
| Realm: | Riboviria |
| Kingdom: | Orthornavirae |
| Phylum: | Negarnaviricota |
| Class: | Monjiviricetes |
| Order: | Mononegavirales |
| Family: | Filoviridae |
| Genus: | Marburgvirus |
| Species: |
Marburg marburgvirus
|
| Virus: |
Marburg virus
|
The Marburg virus (MARV) is a very dangerous virus that causes a serious illness called Marburg virus disease. This disease is a type of hemorrhagic fever, which means it can cause bleeding inside and outside the body. Marburg virus is part of the Filoviridae family, just like the Ebola virus.
Experts consider the Marburg virus to be extremely risky. The World Health Organization (WHO) says it needs the highest level of safety precautions, called biosafety level 4. This is because it's so easy to catch and can be deadly. In the United States, it's listed as a top-priority germ that could be used in bioterrorism.
The virus can spread to people from certain types of fruit bats. It can also spread from person to person through contact with body fluids, like blood or sweat, or through broken skin. The illness causes symptoms similar to Ebola, such as fever and bleeding. Currently, there are no approved vaccines or specific medicines to cure Marburg virus disease. However, getting early medical care, like staying hydrated, can greatly improve a person's chances of survival.
In 2009, scientists started testing a vaccine in Kampala, Uganda, that might protect against both Ebola and Marburg viruses.
Contents
What is Marburg Virus Disease?
Marburg virus disease (MVD) is the illness caused by the Marburg virus. There are actually two types of Marburg viruses that can cause this disease in humans: MARV and Ravn virus (RAVV). Both are very similar to each other.
Past Outbreaks of Marburg Virus
Marburg virus outbreaks have happened in different parts of the world. Here's a look at some of the recorded outbreaks:
| Year | Location | Virus Type | Cases | Deaths | Death Rate | Notes |
|---|---|---|---|---|---|---|
| 1967 | Marburg and Frankfurt, West Germany, and Belgrade, Socialist Federal Republic of Yugoslavia | MARV | 31 | 7 | 23% | This outbreak started from a laboratory accident. |
| 1975 | Rhodesia and Johannesburg, South Africa | MARV | 3 | 1 | 33% | |
| 1980 | Kenya | MARV | 2 | 1 | 50% | |
| 1987 | Kenya | RAVV | 1 | 1 | 100% | |
| 1988 | Koltsovo, Soviet Union | 1 | 1 | 100% | Another laboratory accident. | |
| 1990 | Koltsovo, Soviet Union | MARV | 1 | 1 | 100% | Another laboratory accident. |
| 1998–2000 | Durba and Watsa, Democratic Republic of the Congo | MARV & RAVV | 154 | 128 | 83% | Both MARV and Ravn virus caused illness at the same time. |
| 2004–2005 | Angola | MARV | 374 | 329 | 90% | This was a very large outbreak. |
| 2007 | Uganda | MARV & RAVV | 4 | 1 | 25% | |
| 2008 | Uganda and The Netherlands | MARV | 1 | 1 | 100% | |
| 2012 | Uganda | MARV | 18 | 9 | 50% | |
| 2014 | Uganda | MARV | 1 | 1 | 100% | |
| 2017 | Uganda | MARV | 3 | 3 | 100% | |
| 2021 | Guinea | MARV | 1 | 1 | 100% | This case was found near the borders with Sierra Leone and Liberia. |
| 2022 | Ghana | MARV | 4 | 3 | 75% | |
| February 2023 | Equatorial Guinea | 25 | 11 | 44% | ||
| March 2023 | Tanzania | 8 | 5 | 63% | ||
| September 2024 | Rwanda | 26 | 8 |
How Can We Prevent Marburg Virus?
Scientists are working hard to find ways to prevent Marburg virus disease. The first human study for a Marburg virus vaccine happened in 2014. This study looked at a "DNA vaccine" and found that people who received it developed some antibodies, which are special proteins that fight off germs. However, these vaccines weren't expected to give full protection.
Researchers also test vaccines on animals like hamsters, mice, and monkeys (called "non-human primates" or NHPs). Mice are useful for early tests, but their immune systems are different from humans. Monkeys are often used because their bodies react to the virus in a way that is very similar to humans.
Scientists have tried different types of vaccines. Some, called "virus replicon particles" (VRPs), worked well in guinea pigs but not as well in monkeys. An "inactivated virus vaccine" (made from a killed virus) didn't work at all. DNA vaccines showed some promise in monkeys, but the monkeys still showed signs of infection.
Because Marburg virus and Ebola virus are from the same family, scientists are trying to create one vaccine that works for both. This would make it easier and cheaper to protect people, especially in developing countries. So far, a single vaccine for both viruses hasn't caused any bad reactions compared to getting two separate shots.
As of June 2022, researchers in Canada found good results with a vaccine called PHV01 in guinea pigs. This vaccine, given about a month before infection, offered strong protection.
Even with all this research, there isn't a widely available vaccine for Marburg virus yet. Human trials have either not been fully successful or lack enough information specifically for Marburg. Developing a vaccine is expensive, and because there are relatively few outbreaks compared to other diseases, there hasn't been enough money or interest to create a vaccine for everyone.
See also
 In Spanish: Marburgvirus para niños
In Spanish: Marburgvirus para niños
 | Dorothy Vaughan |
 | Charles Henry Turner |
 | Hildrus Poindexter |
 | Henry Cecil McBay |

