Molecular vibrations facts for kids
Molecular vibrations are one of the ways tiny molecules move. Think of a molecule as a group of atoms connected by springs. These springs can stretch and bend.
Besides vibrating, molecules can also move in two other ways:
- Translational motion: This is when the whole molecule moves from one place to another, like a car driving down a road.
- Rotational motion: This is when the molecule spins around, like a top.
When a molecule vibrates, the bonds (the "springs") between its atoms stretch, squeeze, or bend. For example, in a simple molecule like hydrogen (H2), nitrogen (N2), or oxygen (O2), the two atoms can just stretch away from each other and then come back together.
Contents
How Molecules Vibrate
When a molecule has more than two atoms, its vibrations become more interesting. Let's look at water (H2O), which has three atoms: one oxygen atom in the middle and two hydrogen atoms attached to it.
While a two-atom molecule only has one way to stretch, a water molecule has two ways to stretch and four other ways to vibrate. These are called bending vibrations.
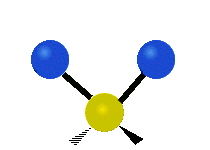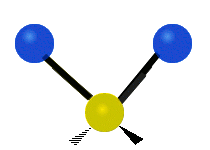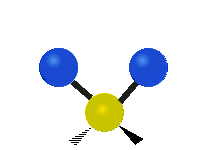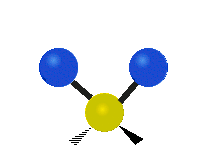
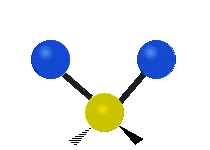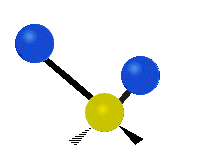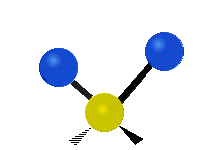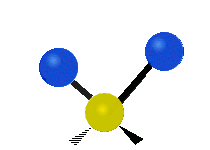
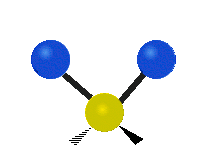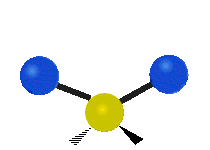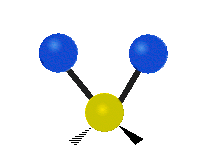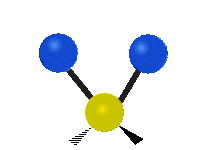
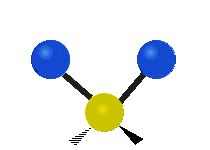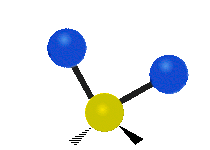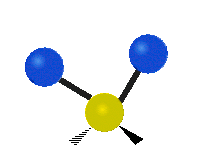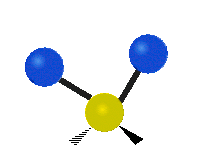
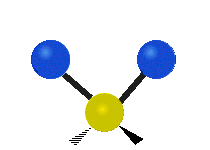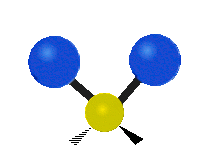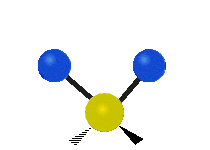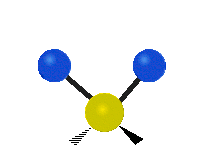
The atoms in a group like CH2 (found in many molecules) or in water can vibrate in six main ways:
| Symmetrical stretching |
Antisymmetrical stretching |
Scissoring |
|---|---|---|
 |
 |
 |
| Rocking | Wagging | Twisting |
 |
 |
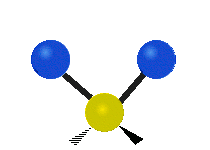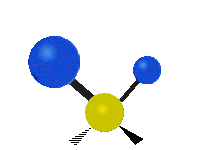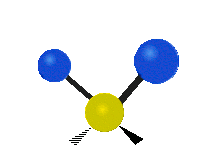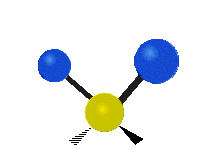 |
- Symmetric stretching: Imagine two atoms attached to a central atom. In symmetric stretching, both attached atoms move away from the central atom at the same time, and then both move back towards it at the same time.
- Antisymmetric stretching: Here, the two attached atoms do not move at the same time. As one moves away from the central atom, the other moves towards it.
- Scissoring: This motion looks like a pair of scissors closing and opening. The two attached atoms move towards each other and then away from each other.
- Rocking: This is like a pendulum swinging back and forth. The two attached atoms move together from side to side, while staying the same distance from the central atom.
- Wagging: Imagine holding up your hand with two fingers in a "V" shape. If your wrist is the central atom and your fingertips are the attached atoms, wagging is like bending your wrist forward and backward. The two attached atoms move together above and below the plane of the molecule.
- Twisting: This motion is like a person walking on a treadmill. If your waist is the central atom and your feet are the two attached atoms, twisting is when one foot moves forward while the other moves backward, and then they switch. The two attached atoms move in opposite directions, twisting around the bond.
Vibrations in Larger Molecules
Molecules with more than three atoms are even more complex. They have many more ways to vibrate, often called "vibrational modes." Each new vibrational mode is usually a combination of the six basic types mentioned above. The more atoms a molecule has, the more ways they can combine to vibrate.
For most molecules with N atoms, there are usually 3N - 6 possible vibrations. However, if the atoms are in a straight line (a linear molecule), they have 3N - 5 vibrational modes.
Energy and Molecular Motion
Bonds as Springs
Scientists can understand molecular vibrations by thinking of the bonds between atoms as tiny springs. Just like a real spring, it takes energy to stretch a bond out or squeeze it together.
The amount of energy needed to make a bond vibrate depends on two main things:
- How stiff the bond is (like how stiff a spring is).
- The combined mass of the two atoms connected by the bond.
When a molecule absorbs energy, its bonds can start to vibrate faster.
Energy Levels
In the world of tiny particles, energy works a bit differently. Think of energy levels like steps on a ladder. A molecule can only have certain amounts of energy, just like you can only stand on a step, not in between steps.
When a molecule vibrates, it can only jump from one energy level to another. It can't have an energy value that's in between these allowed levels. Usually, a molecule can only go up or down one "step" at a time when it gains or loses energy.
How We Use Molecular Vibrations
Molecular vibrations are very useful in science, especially in a field called spectroscopy.
When light of a certain frequency (or color) hits a molecule, and that frequency matches one of the molecule's natural vibration frequencies, the molecule will absorb the light. This absorbed energy makes the molecule's bonds vibrate more strongly.
By shining different frequencies of light on a sample and seeing which ones are absorbed, scientists can figure out what kinds of bonds are present in a molecule. This helps them identify unknown substances or study the structure of known ones.
For example, molecules like helium or argon have only one atom and no bonds. Because they don't have bonds to vibrate, they won't absorb light in the same way molecules with multiple atoms do.
Two important types of spectroscopy that use molecular vibrations are infrared spectroscopy (IR) and Raman spectroscopy.
- Infrared (IR) Spectroscopy: This is a very popular method.
- Near IR: Used to measure things like proteins, fats, and water in food, farm products, and petroleum.
- Mid IR: The most common type of IR, used to figure out the structure of organic (carbon-based) and biological molecules.
- Far IR: Less common, but used for studying inorganic (non-carbon) substances.
- Raman Spectroscopy: Often used along with IR, Raman helps scientists study both inorganic, organic, and biological systems. It's great for identifying and measuring different substances.
Images for kids
See also
 In Spanish: Vibración molecular para niños
In Spanish: Vibración molecular para niños