Neutral oxide facts for kids
Neutral oxides are special kinds of chemical compounds called oxides. An oxide is simply a compound that contains at least one oxygen atom and one other element in its chemical formula. What makes neutral oxides unique is that they don't act like acids or bases. This means they won't react with acids or bases in a typical acid-base reaction. Think of them as the "middle ground" in the world of chemical reactions.
Contents
What Are Neutral Oxides?
Neutral oxides are chemical compounds that contain oxygen and another element, but they show no acidic or basic properties. This means they don't change the color of litmus paper (a common test for acids and bases), and they don't react with strong acids or strong bases to form salts and water. They are quite stable and unreactive in this specific way.
How Do They Behave?
Unlike acidic oxides (which react with bases) or basic oxides (which react with acids), neutral oxides just don't participate in these kinds of reactions. For example, if you mix a neutral oxide with an acid, nothing much will happen. The same goes for mixing it with a base. This non-reactive nature in acid-base chemistry is their defining characteristic.
Neutral vs. Amphoteric: What's the Difference?
It's easy to confuse neutral oxides with amphoteric oxides, but they are different! Amphoteric oxides are compounds that can react as an acid or as a base, depending on what they are mixed with. They are like chemical chameleons. Neutral oxides, however, do not react as either an acid or a base under any conditions. They are truly "neutral" in their acid-base behavior.
Common Examples of Neutral Oxides
While there aren't many neutral oxides known, the ones we do know are quite important in everyday life and in different industries. Here are some of the most common examples:
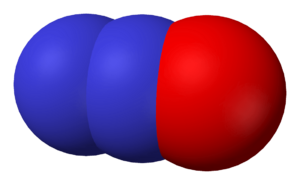
Carbon Monoxide (CO)
Carbon monoxide is a colorless, odorless, and tasteless gas. It's formed when carbon-containing fuels (like wood, coal, or gasoline) don't burn completely. Even though it's very dangerous if inhaled (it can be deadly because it stops your blood from carrying oxygen), it doesn't act as an acid or a base. It's a key component in some industrial processes.
Nitrous Oxide (N₂O)
Nitrous oxide, also known as "laughing gas," is another neutral oxide. It's often used in medicine as an anesthetic (to make people sleep during operations) and to reduce pain. It's also used as a propellant in aerosol cans, like whipped cream dispensers. Despite its various uses, it doesn't show any acidic or basic properties.
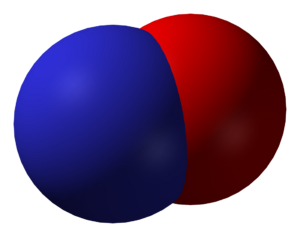
Nitric Oxide (NO)
Nitric oxide is a very important molecule in the human body. It acts as a signaling molecule, helping with things like blood vessel relaxation, which affects blood pressure. It's also involved in the immune system and nerve signaling. In the environment, it's a component of air pollution. Chemically, it's a neutral oxide, meaning it doesn't react as an acid or a base.
Water (H₂O)
You might be surprised to learn that water is considered a neutral oxide! Water is essential for all known forms of life. While water can sometimes act as a very weak acid or a very weak base (it can slightly ionize into H+ and OH- ions), it is overwhelmingly neutral in its overall behavior. It doesn't react strongly with acids or bases in the way that acidic or basic oxides do. Its pH is 7, which is the definition of neutral.
Manganese(IV) Oxide (MnO₂)
Manganese(IV) oxide is a black solid that is commonly found in nature as the mineral pyrolusite. It's widely used in dry-cell batteries (like the ones you put in flashlights) and as a pigment in ceramics and glass. It's also used as a catalyst in some chemical reactions. Despite its many uses, it remains a neutral oxide, showing no acidic or basic properties.
Why Are Neutral Oxides Important?
Understanding neutral oxides helps scientists classify chemical compounds and predict how they will behave in different situations. Even though they don't react with acids or bases, they have important roles in various applications, from medicine to industry, as seen with carbon monoxide, nitrous oxide, and manganese(IV) oxide.
How Many Neutral Oxides Are There?
Scientists have discovered a limited number of neutral oxides. So far, there are only seven neutral oxides known to exist. The five listed above are the most common and well-known examples.