Nickel(II) sulfate facts for kids
Nickel(II) sulfate, also known as nickelous sulfate, is a special chemical compound. Think of it as a tiny building block made from different parts. Its chemical formula is NiSO4. This means it has one nickel atom, one sulfur atom, and four oxygen atoms all linked together. The nickel in this compound has a special electrical charge, called a +2 oxidation state. It also contains sulfate ions, which are groups of sulfur and oxygen atoms with their own charge.
Contents
What is Nickel(II) Sulfate Like?
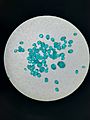
Nickel(II) sulfate is usually a pretty bluish solid. It loves water and can easily dissolve in it, just like sugar dissolves in tea! Most of the time, you'll find it with some water molecules attached to it. When it has these water molecules, we call it "hydrated." But if you take all the water away, it changes color and becomes yellow. This is called being "anhydrous." When it mixes with certain other chemicals called bases, it can turn into a different compound called nickel(II) hydroxide.
How is Nickel(II) Sulfate Made?
Scientists and chemists can make nickel(II) sulfate by mixing two things. They take nickel(II) oxide (which is another nickel compound) or even pure nickel metal, and then they dissolve it in sulfuric acid. Sulfuric acid is a very strong acid, so this process needs to be done carefully in a lab.
What is Nickel(II) Sulfate Used For?
Nickel(II) sulfate is quite useful!
- Making other nickel compounds: It's often used as a starting material to create many other types of nickel chemicals.
- Electroplating: One cool use is in something called electroplating. This is a process where a thin layer of nickel is put onto metal objects. It can make things look shiny or protect them from rust. For example, some tools or car parts might be electroplated with nickel.
- Lab work: It's also used in science labs for different experiments and research.
Related pages
Images for kids
See also
 In Spanish: Sulfato de níquel(II) para niños
In Spanish: Sulfato de níquel(II) para niños
 | Aurelia Browder |
 | Nannie Helen Burroughs |
 | Michelle Alexander |