Peanut allergy facts for kids
Quick facts for kids Peanut allergy |
|
|---|---|
 |
|
| A peanut allergy warning | |
| Symptoms | Itchiness, hives, swelling, eczema, sneezing, asthma attack, abdominal pain, drop in blood pressure, diarrhea, cardiac arrest |
| Causes | Type I hypersensitivity |
| Risk factors | Childhood in developed countries |
| Diagnostic method | Medical history and physical examination by an approved doctor |
| Similar conditions | Tree nut allergy |
| Prevention | Proper early introduction to peanuts and their products during pregnancy and infancy |
| Treatment | Epinephrine Antihistamines (mild) |
| Frequency | 0.6% (US) 1.5–3.0% (Western world) |
Peanut allergy is a type of food allergy to peanuts. It is different from tree nut allergies, because peanuts are legumes (like beans or peas) and not true nuts. When someone with a peanut allergy eats peanuts, their body can react strongly.
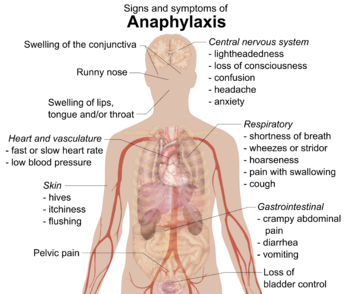
Symptoms can include itchiness, hives (itchy red bumps), swelling, eczema (itchy skin rash), sneezing, asthma attacks, stomach pain, low blood pressure, diarrhea, and even cardiac arrest (when the heart stops). A very serious reaction called Anaphylaxis can happen. People with asthma are more likely to have a severe reaction.
This allergy happens because the immune system (your body's defense system) overreacts to peanut proteins. It's known as one of the most serious food allergies because it's common, often lasts a lifetime, and can cause very severe reactions.
Doctors now suggest that giving babies peanut products early might help prevent this allergy. This is especially true for babies who are at high risk. The main treatment for a severe reaction (anaphylaxis) is an injection of epinephrine.
In the United States, about 0.6% of people have a peanut allergy. In Western countries, between 1.5% and 3% of children have it, and these numbers have been growing. Peanut allergy is a common cause of serious and sometimes deadly allergic reactions from food.
What happens during a peanut allergy reaction?
When someone with a peanut allergy eats peanuts, their immune system sees the peanut proteins as a threat. It releases special chemicals, like histamine. These chemicals cause the body to react in different ways.
For example, histamine can make blood vessels widen and the airways in the lungs get tighter. This can lead to symptoms like mild itchiness, hives, swelling in the face, a runny nose, vomiting, diarrhea, stomach pain, worse atopic eczema, asthma, and even cardiac arrest. The most serious reaction is Anaphylaxis.
Can I be allergic to other foods if I have a peanut allergy?
Yes, sometimes. People with a peanut allergy might also react to tree nuts, soy, or other legumes like peas and lentils. This is called cross-reactivity. It happens because these foods have similar proteins to peanuts.
Studies show that between 6% and 40% of people with a peanut allergy might have allergic symptoms when they eat tree nuts or other legumes.
What causes peanut allergy?
The most common way to get an allergic reaction is by eating peanuts. But sometimes, touching peanuts or breathing in tiny peanut particles can also cause a reaction. Scientists have found at least 11 different peanut proteins that can cause an allergy.
These peanut proteins trigger an immune response in the body. The body creates special Immunoglobulin E (IgE) antibodies. When these antibodies meet peanut proteins, they tell certain cells (called mast cells) to release chemicals that cause the allergic reaction.
Peanut allergies are not common in children in poorer countries where peanuts are often used to help with malnutrition. One idea, called the hygiene hypothesis, suggests that being exposed to peanuts early in life might help the immune system learn not to overreact.
Can early exposure prevent peanut allergy?
Yes! Studies have shown that giving peanut proteins to babies between 4 and 11 months old can greatly lower their risk of developing a peanut allergy. Because of this, doctors now suggest that parents do not need to avoid giving peanuts to their babies, even if there's a family history of allergies. They also say there's no reason to avoid peanuts during pregnancy or while breastfeeding.
One important study gave small amounts of peanut flour daily to children who were at high risk for allergies. A large number of these children became less sensitive to peanuts, showing that early exposure can help prevent the allergy.
Does what a pregnant person eats affect allergies?
There isn't enough clear evidence yet to say if what a pregnant person eats affects whether their child develops allergies. More research is needed on this topic.
How can someone be exposed to peanuts?
Most allergic reactions happen when someone accidentally eats peanuts. But sometimes, reactions can happen from touching peanuts or breathing in tiny bits of them. Peanut allergens can stay in the environment for months.
For example, tiny peanut particles in the air from a factory or from cooking can cause breathing problems for allergic people. However, some reports of severe reactions from just being near peanuts have been shown to be exaggerated. Touching peanut residue on surfaces might cause a minor skin rash, but usually not a severe reaction like anaphylaxis.
Some reactions might even be caused by a person's mind, not a true chemical reaction. Studies where people didn't know if they were near peanuts or not showed that just the smell of peanut butter or being close to it didn't cause reactions.
Very rarely, allergic reactions have been triggered by kissing someone who recently ate peanuts.
How do doctors diagnose peanut allergy?
Diagnosing a food allergy, including peanut allergy, usually starts with a doctor asking about your health history and doing a physical exam. It's important for a doctor to confirm a food allergy, because many people think they have an allergy when they actually don't.
Skin prick testing
Doctors can use skin prick tests to help confirm specific food allergies. In this test, a tiny drop of liquid with peanut protein is placed on your skin. Then, the skin is gently pricked through the drop. If you're allergic, a small, itchy bump (like a mosquito bite) will appear.
This test can help, but it's not always 100% accurate. A positive skin prick test alone doesn't always mean you have a food allergy.
Oral food challenge
The best way to diagnose a food allergy is with an "oral food challenge." This is done under close medical supervision. Before the test, you avoid the suspected food for at least two weeks.
During the challenge, you are given very small, increasing amounts of the suspected food. Doctors watch you carefully for any signs of an allergic reaction. If a reaction starts, they stop the test and give you treatment.
Oral food challenges can be risky because they can cause allergic reactions. Some reactions can be severe. These tests also take a lot of time and need careful medical supervision.
How can peanut allergy be prevented?
In 2017, health experts in the U.S. released new guidelines for preventing peanut allergies in babies. They suggest different ways to introduce peanut foods to infants, depending on their risk level.
For babies at high risk (for example, if they have severe eczema or another food allergy), an allergy specialist might do tests first. Then, peanut foods are slowly introduced, often with the specialist watching. For babies with moderate risk (like mild to moderate eczema), parents can slowly introduce peanut foods at home, starting around 6 months of age.
A big study called LEAP (Learning Early About Peanut Allergy) showed that introducing peanut products early into a child's diet can prevent peanut allergies, not just delay them. This benefit seems to last a long time.
How is peanut allergy treated?
If someone with a peanut allergy accidentally eats peanuts, the treatment depends on how sensitive they are. For mild reactions, an antihistamine medicine might be given. Sometimes, a medicine called prednisone is prescribed to prevent a delayed reaction.
For severe allergic reactions (anaphylaxis), an epinephrine pen is used. This is a special injection device that people can use themselves or have someone else use on them in an emergency.
As of 2021, there is no cure for peanut allergy. The best way to manage it is to strictly avoid peanuts and foods that contain them. It's important to be extra careful when eating out or buying food from restaurants.
Immunotherapy
Immunotherapy is a way to try and reduce allergic reactions by slowly exposing a person to tiny amounts of peanut products over time. However, as of 2019, some studies have found that this might actually increase the risk of serious allergies. These treatments are still being studied and are not yet widely used outside of special trials.
In 2020, a peanut allergen powder (called Palforzia) was approved in the United States. It's used to help reduce allergic reactions, including anaphylaxis, that might happen from accidental peanut exposure. This treatment can be started in people aged 4 to 17 years old. Even with this treatment, people still need to avoid peanuts in their diet.
How common is peanut allergy?
About 0.6% of people in the United States have a peanut allergy. In Western countries, between 1.5% and 3% of children have it. The number of cases has increased a lot in the early 21st century.
Peanut allergy is one of the most serious food allergies and one of the least likely for children to outgrow. It is a common cause of deaths related to food allergies. However, some people believe that the fear around peanut allergies might be bigger than the actual danger.
The number of children who say they have a peanut allergy has doubled between 1997 and 2002. But studies show that the number of people who report having food allergies is often higher than the number actually confirmed by doctors.
How peanut allergy affects daily life
Because peanut allergy can cause very serious reactions and is becoming more common, it gets a lot of public attention. This has changed daily life for children with the allergy, their parents, and caregivers.
In the United States, a law passed in 2004 (FALCPA) makes food companies put allergy warnings on packaged foods. Restaurants have also added allergy warnings to their menus. Many schools have rules about what foods can be brought in to keep allergic students safe.
Even with all these precautions, people with serious allergies know that accidental exposure can still happen at friends' houses, at school, or in restaurants. The fear of food can really affect a person's quality of life. Also, children with allergies can sometimes face bullying, where others might threaten them or even try to contaminate their food.
Food Labels and Allergies
To help people with food allergies, many countries have laws that require food labels to clearly state if a product contains major allergens.
Ingredients that are added on purpose
In the United States, the Food Allergen Labeling and Consumer Protection Act of 2004 (FALCPA) requires companies to list eight major food allergens if they are added on purpose: cow's milk, peanuts, eggs, shellfish, fish, tree nuts, soy, and wheat. If an ingredient comes from one of these allergens, it must be clearly stated (e.g., "Casein (milk)"). The European Union has a similar law but includes more allergens.
These laws apply to packaged foods. They don't usually apply to food made in restaurants or to medicines and cosmetics.
Tiny amounts from cross-contamination
Sometimes, tiny amounts of an allergen can get into food accidentally during manufacturing (called cross-contamination). Labeling for these trace amounts is more complicated. Some labels might say "may contain" (called precautionary allergen labeling or PAL). This can be confusing because the wording can be different, and it doesn't always mean the allergen is definitely there.
Some countries are working on better rules for these "may contain" labels to make them clearer for consumers. For example, Brazil requires companies to state if there's a possibility of cross-contamination if they can't prevent it.
See also
 In Spanish: Alergia al cacahuete para niños
In Spanish: Alergia al cacahuete para niños