Mustard gas facts for kids
| Sulfur mustard (HD) | |
|---|---|
| General | |
| Systematic name | Bis (2-chloroethyl) sulfide |
| Other names | Iprit Kampfstoff "Lost" Lost Mustard gas Senfgas Yellow Cross Liquid Yperite |
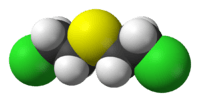
| Molecular formula | C4H8Cl2S |
| Molar mass | 159 g/mol |
| Appearance | Colorless if pure. Normally ranges from pale yellow to dark brown. Slight garlic type odor. |
Mustard gas is also known as sulfur mustard. It is a special kind of chemical compound. People have used it as a chemical weapon in wars. This means it's a harmful chemical used to hurt or kill people.
Mustard gas was first used in World War I. The German Army used it against British and Canadian soldiers. This happened near a town called Ypres in Belgium in 1917. Later, it was also used against the French army.
When it's pure, sulfur mustard is a clear liquid with no smell. But when it's used in war, it often looks yellowish to brown. It can sometimes smell a bit like culinary mustard, horseradish, or garlic. This is how it got its name, even though it's not related to the food.
What is Mustard Gas?
Sulfur mustard is a chemical compound with the formula (ClCH2CH2)2S. It's a thick liquid when it's at normal room temperature. The pure chemical melts at 14 °C (57 °F). It breaks down before it can boil at 218 °C (424 °F).
This chemical is very dangerous. It can cause serious burns and blisters on skin. It also harms eyes and lungs. This is why it is called a "blister agent."
History and How It Was Used
A scientist named Frederick Guthrie made sulfur mustard in 1860. Some people think it might have been found even earlier, around the 1820s.
Its use in warfare was banned by the Geneva Protocol of 1925. This important agreement made it illegal to use poison gas. Poison gas was used a lot during the First World War.
Another agreement, called the Chemical Weapons Convention, was made in 1993. This agreement also made it illegal to make or store chemical weapons like mustard gas. These rules help keep the world safer from such dangerous chemicals.
Images for kids
-
Pallets of 155 mm artillery shells containing "HD" (distilled mustard gas agent) at the Pueblo Chemical Depot. The distinctive color-coding scheme on each shell is visible
See also
 In Spanish: Gas mostaza para niños
In Spanish: Gas mostaza para niños