Tetrasulfur tetranitride facts for kids
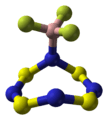
Tetrasulfur tetranitride, also known as tetrasulphur tetranitride, is a special chemical compound. Its chemical formula is S4N4. This means it's made of four sulfur atoms and four nitrogen atoms. In this compound, sulfur has a +3 oxidation state, and it also contains nitride ions.
Contents
What it Looks Like and How it Behaves
Tetrasulfur tetranitride is a shiny gold solid. It can explode! When it does, it breaks down into harmless sulfur and nitrogen. The purer this substance is, the more easily it can explode.
Its color changes with temperature:
- When it's cold, it looks pale yellow.
- At normal room temperature, it's orange.
- When it gets hot, it turns red.
This compound reacts with certain chemicals called bases. If you mix it with silver, it can change into another chemical called disulfur dinitride. It doesn't dissolve in water.
How it's Made
Scientists can make tetrasulfur tetranitride in a couple of ways:
- One way is by mixing disulfur dichloride with ammonia.
- Another way is to mix ammonium chloride with disulfur dichloride.
What it's Used For
Scientists use tetrasulfur tetranitride to react with many different organic compounds. These are chemicals that are often found in living things or are made from carbon.
Staying Safe with it
This chemical is explosive, so it's very important not to grind it or handle it roughly. If it does explode, it turns into harmless nitrogen gas and sulfur.
Related Pages
Images for kids
See also
 In Spanish: Tetranitruro de tetraazufre para niños
In Spanish: Tetranitruro de tetraazufre para niños