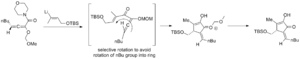Torquoselectivity facts for kids
Torquoselectivity is a cool idea in organic chemistry! It helps us understand how certain chemical reactions, called electrocyclic reactions, create specific shapes of molecules.
Imagine you have a ring of atoms, and some other atoms or groups are attached to it. During an electrocyclic reaction, this ring might open up, or a straight chain of atoms might close to form a ring. As this happens, the groups attached to the ends of the chain or ring will rotate.
Torquoselectivity means that these attached groups prefer to rotate in one specific direction (either inwards or outwards) rather than just randomly. Because of this preferred rotation, the reaction mostly makes one specific product, instead of a mix of different ones. It's like a chemical dance where the dancers always turn left, not right! This idea was first developed by a scientist named Kendall N. Houk.
Contents
How Rings Form: Torquoselectivity in Action
When a chemical reaction forms a ring, we call it a ring-closing reaction. Torquoselectivity plays a big part here.
Choosing the Right Shape
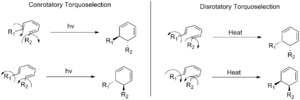
In these reactions, the atoms can rotate in two main ways:
- Conrotatory: Both ends of the molecule rotate in the same direction (e.g., both clockwise or both counter-clockwise).
- Disrotatory: The ends rotate in opposite directions (e.g., one clockwise, one counter-clockwise).
Even with these choices, a reaction can still make two slightly different versions of the same molecule, called enantiomers. Torquoselectivity helps the reaction pick just one of these enantiomers. This is important because in chemistry, the exact shape of a molecule can change how it works!
When Rings Open: Another Look at Torquoselectivity
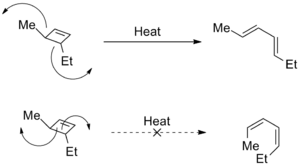
Torquoselectivity also happens when electrocyclic reactions break open rings. In these cases, the different ways the atoms rotate can lead to completely different molecules, called structural isomers.
Why Rings Open a Certain Way
Often, the main reason for this specific rotation is to avoid steric strain. This means the atoms try to move in a way that avoids bumping into each other too much. Imagine trying to open a door in a crowded room – you'd turn the handle in the direction that gives you the most space!
Scientists have also found that adding certain types of chemical groups to the molecule can change how it rotates. Some groups, called electron-donating groups, give away electrons, while electron-withdrawing groups pull electrons towards them. Both types can influence the rotation.
Other Ways Torquoselectivity Works
Torquoselectivity isn't just about simple ring openings and closings. It can also be influenced by other things in a chemical reaction.
Help from Catalysts
Sometimes, special chemicals called Lewis acid catalysts can guide the rotation. If these catalysts are chiral (meaning they have a "handedness" like your left and right hands), they can help the reaction make only one specific product shape.
Neighboring Atoms and Chirality Transfer
Nearby atoms in a molecule, especially those with a specific 3D arrangement (called stereocenters), can also influence torquoselectivity. This is a type of diastereoselectivity, where the reaction prefers one specific shape over another.
Another cool way torquoselectivity works is through "axial-to-tetrahedral chirality transfer." This is a fancy way of saying that the 3D shape of one part of a molecule can influence the 3D shape of another part as the reaction happens. An example of this is seen in a reaction called the Nazarov cyclization reaction.