Woodward's rules facts for kids
Woodward's rules are a set of guidelines that help scientists understand how certain organic chemicals soak up ultraviolet (UV) light.
These rules help predict the exact wavelength of light where a chemical compound absorbs the most UV light. This is called the absorption maximum, shown as λmax on a spectrum. The rules are named after Robert Burns Woodward, a professor from Harvard University. He won the Nobel Prize in Chemistry in 1965. Sometimes, these rules are also called the Woodward-Fieser rules to also honor Louis Fieser.
The rules work by looking at special parts of a molecule called chromophores. These are the parts that absorb light. They also consider other small groups attached to the molecule (called substituents) and how the solvent (the liquid the chemical is dissolved in) affects it. Examples of chemicals these rules work for include conjugated carbonyl compounds (like ketones or aldehydes), and dienes or polyenes (molecules with many double bonds).
How Woodward's Rules Work
One common set of Woodward-Fieser rules is used for dienes. Dienes are molecules that have two double bonds. These double bonds can be in the same ring (called homoannular) or in different rings (called heteroannular).
The rules use a starting number, called a "base value," and then add extra numbers (called "increments") for different features of the molecule. This helps predict the λmax.
| Base value for heteroannular diene | 214 |
| Base value for homoannular diene | 253 |
| Extra Numbers to Add | |
| Double bond that extends the chain of double bonds | + 30 |
| Alkyl group (like a methane or ethane part) or a ring part | + 5 |
| Exocyclic double bond (a double bond that is part of a ring but also sticks out from another ring) | + 5 |
| acetate group | + 0 |
| Ether group (an oxygen atom connected to two carbon groups) | + 6 |
| Thioether group (a sulfur atom connected to two carbon groups) | + 30 |
| bromine or chlorine atom | + 5 |
| Secondary amine group (a nitrogen atom connected to two carbon groups and one hydrogen) | + 60 |
| Table 1. Rules for the wavelength of maximum diene absorption (in nanometers) |
|
These rules help scientists guess the UV absorption maximum for many compounds. Let's look at two examples:
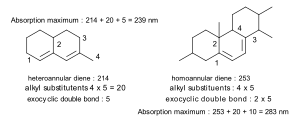
In the molecule on the left, the starting value is 214 nm because it's a heteroannular diene (the two double bonds are in different rings). This diene has four alkyl groups attached (marked 1, 2, 3, 4). Also, one of its double bonds is "exocyclic" to the other ring, which adds 5 nm.
For the molecule on the right, the starting value is 253 nm because it's a homoannular diene (both double bonds are in the same ring, ring B). This molecule also has four alkyl groups attached. Both double bonds in the middle ring (B) are exocyclic to the rings next to them (rings A and C).
See also
In Spanish: Reglas de Woodward-Fieser para niños
 | Janet Taylor Pickett |
 | Synthia Saint James |
 | Howardena Pindell |
 | Faith Ringgold |