Carbonyl facts for kids
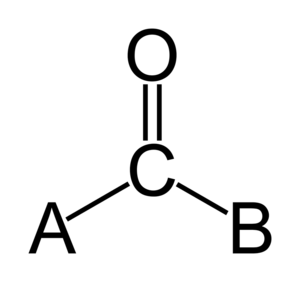
A carbonyl is a special part of many molecules. It's made of one carbon atom and one oxygen atom connected by a double bond. Think of it like two hands holding each other very tightly!
The carbon atom in a carbonyl group still has two other "hands" (or bonds) free to connect to other atoms or groups of atoms. This is why you find carbonyls in many different types of chemicals, like ketones, aldehydes, and esters.
Contents
What Makes Carbonyls Special?
The oxygen atom in a carbonyl group is a bit of an "electron hog." This means it pulls the electrons in the double bond closer to itself than the carbon atom does. Because of this, the oxygen end of the carbonyl group has a slightly negative charge, and the carbon end has a slightly positive charge. This difference in charge makes carbonyls very reactive and useful in chemistry.
How Chemists Use Carbonyls
Chemists love carbonyl groups because they are very easy to work with. You can do many chemical reactions with them:
- Adding new parts: It's easy to add new atoms or groups to the carbon atom of a carbonyl. This is called a nucleophilic addition reaction, and it's a great way to make bigger, more complex molecules.
- Changing them into alcohols: You can also change a carbonyl group into an alcohol group using a type of reaction called a redox reaction. Alcohols are another very important type of chemical group.
Where Are Carbonyls Found?
Carbonyl groups are everywhere!
- They are found in many things that exist naturally, like in sugars, fats, and proteins that make up living things.
- They are also a key part of many drugs and medicines we use every day.
How to Spot a Carbonyl
Scientists have special ways to tell if a molecule has a carbonyl group:
- Infrared Spectroscopy: If you shine infrared light on a molecule, a carbonyl group will give a very strong and clear signal. It's like a bright light flashing, telling you it's there!
- NMR Spectroscopy: In another test called carbon NMR spectroscopy, the signal from a carbonyl group usually shows up in a very specific spot, far away from other signals. This makes it easy to identify.
See Also
- In Spanish: Grupo carbonilo para niños
 | Georgia Louise Harris Brown |
 | Julian Abele |
 | Norma Merrick Sklarek |
 | William Sidney Pittman |