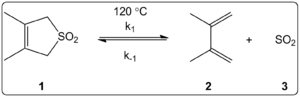Cheletropic reaction facts for kids
A cheletropic reaction is a special type of chemical reaction. It's a kind of pericyclic reaction. In these reactions, one atom from a molecule forms two brand new bonds to another molecule. Think of it like one atom reaching out and grabbing two spots on another molecule at the same time!
Pericyclic reactions are unique because the atoms and their orbitals arrange themselves in a circle for a moment. This helps them swap around their bonds.
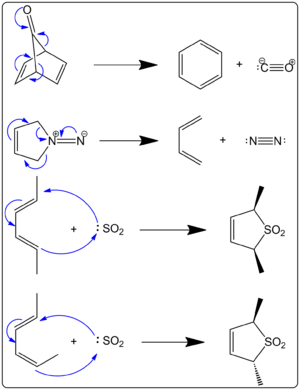
Cheletropic reactions are also a type of cycloaddition. What makes them special is that both new bonds are made to the *same* atom on one of the reacting molecules. Look at Figure 1. The first two examples show "cheletropic extrusions." This means a small, stable molecule like carbon monoxide (CO) or nitrogen gas (N2) is released. This release of gas often helps the reaction happen.
Contents
Cheletropic Reactions with Sulfur Dioxide (SO2)
How Reactions Happen (Thermodynamics and Kinetics)
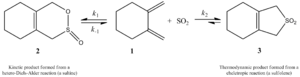
When sulfur dioxide (SO2) reacts with molecules like butadiene, it can make different products. Scientists study how these reactions happen. They look at two main things:
- Kinetic product: This is the product that forms fastest.
- Thermodynamic product: This is the most stable product, meaning it's usually the one you get more of in the end.
For SO2 reactions, the cheletropic reaction often leads to a more stable, five-membered ring. This is the thermodynamic product. The image below shows how one molecule can react to form either a kinetic product (right path) or a more stable thermodynamic product (left path). Scientists like Suarez and Sordo showed this in 1995.
How Fast Reactions Go (Kinetics)
Scientists have studied how fast cheletropic reactions happen. For example, in 1976, Isaacs and Laila looked at how fast sulfur dioxide adds to different butadiene molecules. They found that:
- If the butadiene had groups of atoms that pull electrons away, the reaction slowed down.
- Larger groups of atoms (called "bulky groups") on the butadiene made the reaction go faster. This is because these bulky groups help the molecule get into the right shape for the reaction.
Later, in 2002, Monnat, Vogel, and Sordo studied the reaction of sulfur dioxide with another molecule called 1,2-dimethylidenecyclohexane. They found that this reaction could make two different products depending on the temperature:
- At very cold temperatures (below -60 °C), it made a "sultine" (the kinetic product).
- At warmer temperatures (above -40 °C), it made a "sulfolene" (the thermodynamic product), which is more stable.
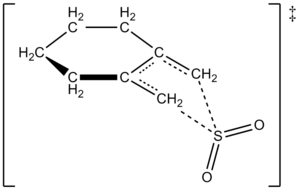
They also discovered something interesting about how fast the sulfolene forms. The reaction rate depends on the amount of 1,2-dimethylidenecyclohexane and *twice* the amount of sulfur dioxide. This means two molecules of sulfur dioxide are involved in the key step of the reaction. One SO2 molecule reacts, and another SO2 molecule helps stabilize the reaction as it happens.
How Solvents Affect Reactions
The liquid a reaction happens in, called the solvent, can also affect how fast a cheletropic reaction goes. Scientists studied a specific cheletropic reaction in 14 different solvents. They found that the reaction speed changed depending on how "polar" the solvent was. A polar solvent is like water, which has positive and negative ends.
Here's what they learned:
- The speed of the forward reaction (making the product) slightly slowed down in more polar solvents.
- The speed of the reverse reaction (product turning back into reactants) sped up a lot in more polar solvents.
- Overall, the balance between reactants and products changed a lot with different solvents.
This suggests that the "polarity" of the molecules changes as they react. This change in polarity is affected by the solvent.
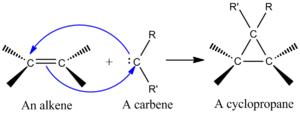
Carbene Additions to Alkenes
One very important cheletropic reaction is when a molecule called a "singlet carbene" adds to an alkene. This reaction makes a cyclopropane, which is a small ring of three carbon atoms.
What is a carbene? It's a special molecule with a carbon atom that only has six electrons around it. This makes carbenes very reactive. They are often formed for a short time during a reaction.
Singlet carbenes add to alkenes in a very specific way. If the alkene has a certain shape, the cyclopropane product will keep that same shape. This reaction is like a [2+1] cycloaddition, where the carbene adds in one step.
Scientists can use carbenes from chemicals like chloroform to add two chlorine atoms to an alkene, making a dihalocyclopropane. Or, they can use something called the Simmons-Smith reagent to add a simple CH2 group.
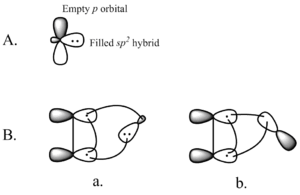
The way the carbene's orbitals interact with the alkene's orbitals helps the reaction happen. It's best if the carbene approaches the alkene at a slight angle, not straight on. This helps the orbitals mix well and form the new bonds.
See also
 In Spanish: Reacción quelotrópica para niños
In Spanish: Reacción quelotrópica para niños