Molecular orbital facts for kids
In chemistry, a molecular orbital (or MO) helps us understand what happens to tiny particles called electrons when atoms join to form a molecule. Think of a molecular orbital as a special map. This map shows where an electron is most likely to be found inside a molecule.
Chemists use these "maps" to guess or explain how chemicals behave. They can also predict their properties. Scientists often create these molecular orbital maps by combining maps of individual atomic orbitals. These are like the electron maps for single atoms.
Computers help a lot with these maps. They allow chemists to use quantum mechanics to study molecules. Molecular orbitals help answer how atoms stick together in molecules. The rounded shapes you see in diagrams show where electrons are most likely to be.
Contents
History of Molecular Orbitals
The word orbital was first used in English by Robert S. Mulliken. But a German physicist named Erwin Schrödinger wrote about these ideas even earlier. He called them Eigenfunktion.
Physicist Max Born explained the main idea behind molecular orbitals in 1926. This idea is now called Born's rule. It is a key part of how we understand quantum mechanics. At first, this idea didn't fit with Niels Bohr's atom model. Bohr's model said electrons moved in circles around the nucleus.
However, Born's model became very popular. It could explain where electrons were inside molecules. It also helped explain many chemical reactions that scientists couldn't understand before.
Understanding Molecular Orbitals
Atomic orbitals help us guess where an electron is in a single atom. When atoms come together, their atomic orbitals combine. This creates molecular orbitals. A molecular orbital tells us about the electron configuration of a molecule. This means it shows the most likely place and energy of an electron (or a pair of electrons).
Often, chemists use a method called the linear combination of atomic orbitals (LCAO-MO method). This is a simpler way to think about it. It means we assume that the chance of finding an electron at any spot in a molecule is the sum of the chances from each individual atomic orbital. This LCAO-MO method is a basic way to understand how atoms bond in molecules. It is very important for studying molecular orbital theory.
Theoretical chemists use computers to figure out the molecular orbitals for different molecules. These can be real molecules or ones they imagine. The computer can draw pictures of the "electron cloud." This cloud shows how likely an electron is to be in any area. Computers can also give information about the physical properties of the molecule. They can even tell how much energy is needed to form the molecule. This helps chemists decide if small molecules can be combined to make bigger ones.
Most modern ways of doing computational chemistry start by calculating the molecular orbitals. Each molecular orbital is affected by the nuclei of all the atoms. It is also affected by the average spread of the other electrons.
An Easy Way to Think About It
Understanding molecular orbitals is a bit like trying to know where every employee is in a big store. Imagine you can't look inside the store. You know how many employees work there and which department each belongs to. You also know employees don't bump into each other. They walk in the aisles, not through the merchandise shelves. Employees might leave their own department to help customers or check inventory.
If you try to guess where all employees are at one moment without looking, it's like a chemist calculating molecular orbitals. Just as molecular orbitals can't tell the exact spot of each electron, you won't know the exact spot of each employee.
A molecular orbital might have a "nodal plane." This is like knowing employees walk down aisles and not through shelves. Even though electrons come from a specific atom, an electron fills a molecular orbital without caring where it came from. This is like an employee leaving their department to walk elsewhere in the store. So, a molecular orbital is a way to describe an electron, just as your guess about the unseen store is a guess about employee locations.
How Molecular Orbitals Form
Chemists have rules for calculating molecular orbitals. These rules come from understanding quantum mechanics. Quantum mechanics helps chemists use what physics says about electrons. This helps them figure out how electrons act in molecules.
Molecular orbitals form from "allowed" interactions between atomic orbitals. These interactions are "allowed" if the symmetries of the atomic orbitals match up. Chemists study how atomic orbitals interact. These interactions happen when atomic orbitals "overlap." Overlap is a way to measure how well two orbitals work together. Overlap is important if the atomic orbitals have similar energy levels.
Finally, the number of molecular orbitals in a molecule must be the same as the number of atomic orbitals that came together to form the molecule.
Simple Approach to Molecular Orbitals
Chemists need to understand the shapes of molecular orbitals. This helps them talk about how molecules are built. The LCAO (Linear combination of atomic orbitals molecular orbital) method gives a simple but good picture of molecular orbitals. In this method, molecular orbitals are shown as combinations of all the atomic orbitals from each atom in the molecule.
Combining Atomic Orbitals (LCAO)
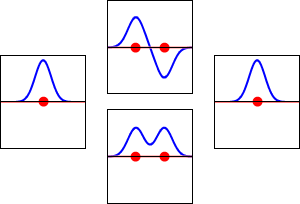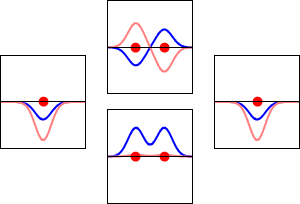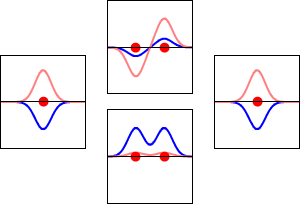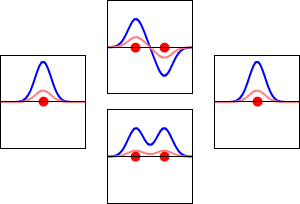
The idea of molecular orbitals was first introduced by Friedrich Hund and Robert S. Mulliken in 1927 and 1928.
The LCAO (linear combination of atomic orbitals) idea for molecular orbitals came from Sir John Lennard-Jones in 1929. His important paper showed how to figure out the electron structure of fluorine and oxygen molecules. This simple way of looking at molecular orbital theory was the start of modern quantum chemistry.
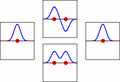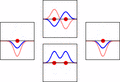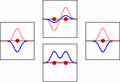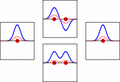
LCAO can help us guess the molecular orbitals that form when atoms bond. Just like an atomic orbital, a Schrödinger equation can be made for a molecular orbital. This equation describes how an electron behaves. LCAO uses sums and differences of the atomic "wavefunctions." These give approximate answers to the molecular Schrödinger equations. For simple molecules with two atoms, the wavefunctions look like these equations:
Ψ = caψa + cbψb
and
Ψ* = caψa - cbψb
Here, Ψ and Ψ* are the molecular wavefunctions. Ψ is for bonding molecular orbitals, and Ψ* is for antibonding ones. ψa and ψb are the atomic wavefunctions from atom 'a' and atom 'b'. The 'c' values (ca and cb) are numbers that can be positive or negative. They depend on the energy and symmetry of the individual atomic orbitals.
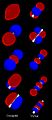
As two atoms get closer, their atomic orbitals overlap. This creates areas where electrons are very likely to be found. So, molecular orbitals form between the two atoms. The atoms are held together by the pull between the positively charged nuclei and the negatively charged electrons in the bonding molecular orbitals.
Bonding, Antibonding, and Nonbonding MOs
When atomic orbitals interact, the new molecular orbital can be one of three types: bonding, antibonding, or nonbonding.
- Bonding MOs:
* These interactions are constructive. This means they add up in a helpful way. * Bonding MOs have lower energy than the atomic orbitals that made them. This makes the molecule more stable.
- Antibonding MOs:
* These interactions are destructive. They cancel each other out. * Antibonding MOs have higher energy than the atomic orbitals that made them. They make the molecule less stable.
- Nonbonding MOs:
* These happen when atomic orbitals don't interact at all. This is because their symmetries don't match. * Nonbonding MOs have the same energy as the atomic orbitals they came from.
HOMO and LUMO
Each molecular orbital has its own energy level. Chemists arrange the molecular orbitals by their energy levels. Electrons will fill the lowest energy level molecular orbitals first. For example, if a molecule has enough electrons to fill 15 orbitals, the 15 molecular orbitals with the lowest energy will be filled.
The 15th molecular orbital on this list would be called the "highest occupied molecular orbital" (HOMO). The 16th molecular orbital (the next one, which is empty) would be the "lowest unoccupied molecular orbital" (LUMO).
The difference in energy between the HOMO and the LUMO is called the band gap. This band gap can sometimes tell us how easily a molecule can get excited. A smaller energy gap means it's easier to excite the molecule. When an electron gets excited, it jumps to an empty molecular orbital. This can help us guess if something will give off light (luminescence).
Images for kids
See also
 In Spanish: Orbital molecular para niños
In Spanish: Orbital molecular para niños