Cyclopropane facts for kids
Quick facts for kids Cyclopropane |
|
|---|---|
|
Preferred IUPAC name
Cyclopropane
|
|
| Identifiers | |
| CAS number | |
| PubChem | |
| KEGG | D03627 |
| ChEBI | CHEBI:30365 |
| SMILES | C1CC1 |
|
InChI
InChI=1/C3H6/c1-2-3-1/h1-3H2
|
|
| Properties | |
| Molecular formula | |
| Molar mass | 0 g mol-1 |
| Appearance | Colorless gas |
| Odor | Sweet smelling |
| Density | 1.879 g/L (1 atm, 0 °C) |
| Melting point | |
| Boiling point | |
| Acidity (pKa) | ~46 |
| -39.9·10−6 cm3/mol | |
| Hazards | |
| Main hazards | Highly flammable Asphyxiant |
| NFPA 704 |
|
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
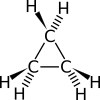
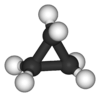
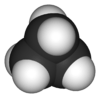
Cyclopropane is a special kind of molecule called a cycloalkane. Its formula is C3H6. This means it has three carbon atoms connected in a ring shape. Each carbon atom is also connected to two hydrogen atoms.
Because the ring is so small, the atoms are a bit squished. This can cause something called ring strain. It makes the molecule less stable than larger rings.
Contents
What is Cyclopropane?
Cyclopropane is a colorless gas. It has a slightly sweet smell. You can't see it, but it's there! Its chemical formula, C3H6, tells us it's made of carbon and hydrogen atoms.
How it Looks and Behaves
The three carbon atoms in cyclopropane form a triangle. This unique shape makes it different from other similar molecules. It has a molar mass of 42.08 grams per mole. This is how much a certain amount of the substance weighs.
It boils at a very cold temperature, around -33 degrees Celsius. It melts at an even colder temperature, -128 degrees Celsius. This shows it's a gas at normal room temperatures.
Past Uses in Medicine
For a long time, cyclopropane was used as an anesthetic. This means it could make people go to sleep during medical procedures. Doctors would have patients inhale the gas.
It worked very quickly and helped patients recover fast. However, it is also very reactive. This means it can easily explode when mixed with oxygen. Because of this danger, doctors stopped using it. Today, other safer chemicals are used for anesthesia.
Safety Information
Cyclopropane is considered highly flammable. This means it can easily catch fire. It can also act as an asphyxiant. An asphyxiant is a substance that can cause you to lose consciousness or even suffocate if there isn't enough oxygen.
Because of these dangers, cyclopropane is handled with extreme care. It is not something you would find or use outside of very specific scientific or industrial settings.
Images for kids
See also
 In Spanish: Ciclopropano para niños
In Spanish: Ciclopropano para niños
 | Misty Copeland |
 | Raven Wilkinson |
 | Debra Austin |
 | Aesha Ash |