Chlorofluorocarbon facts for kids
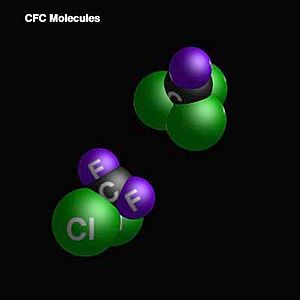
Chlorofluorocarbons (CFCs) are special gases made by people. They contain carbon, chlorine, and fluorine. Sometimes they also have hydrogen.
CFCs were very popular in the mid-1900s. They were used in many products like aerosol sprays, refrigerators, and cleaning liquids. People liked them because they were safer than older chemicals. The older chemicals could be poisonous or catch fire easily.
However, scientists later found that CFCs cause big problems for our planet. They harm the ozone layer and make global warming worse. Because of this, many countries have now banned CFC products. Sweden was the first country to ban them in 1978. The United States and Canada followed soon after.
Today, we use other chemicals instead of CFCs. One example is hydrofluorocarbons (HFCs). These are much better for the environment. They do not damage the ozone layer or add to global warming.
Contents
What are CFCs?
CFCs are a type of chemical compound. They are made of carbon, chlorine, and fluorine atoms. They are gases at room temperature. This makes them useful for many things.
How were CFCs used?
CFCs were used in many everyday items.
- Refrigerators and Air Conditioners: They helped keep things cool.
- Aerosol Sprays: They pushed out the product from the can. Think of hairspray or spray paint.
- Cleaning Products: They were good for cleaning electronic parts.
- Fire Extinguishers: Some types helped put out fires.
Why are CFCs harmful?
CFCs cause two main environmental problems. They damage the ozone layer and act as powerful greenhouse gases.
Damaging the Ozone Layer
The ozone layer is like Earth's natural sunscreen. It is high up in our atmosphere. This layer protects us from the Sun's harmful UV rays. UV rays can cause skin cancer and harm plants.
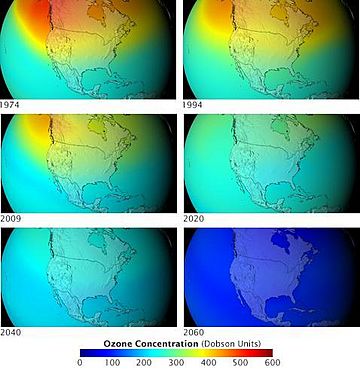
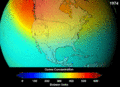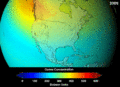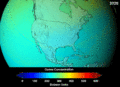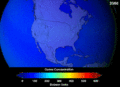
When CFCs get into the atmosphere, they float up to the ozone layer. There, the Sun's strong light breaks them apart. This releases chlorine atoms. These chlorine atoms then destroy ozone molecules. One chlorine atom can destroy thousands of ozone molecules! This creates "holes" in the ozone layer. This lets more harmful UV rays reach Earth.
Contributing to Global Warming
CFCs are also very strong greenhouse gases. Greenhouse gases trap heat in Earth's atmosphere. This causes the planet to warm up. This warming is known as global warming. While carbon dioxide is the main greenhouse gas, CFCs are much more powerful at trapping heat, even in small amounts.
The Ban on CFCs
Scientists discovered the harm from CFCs in the 1970s. This led to worldwide action.
The Montreal Protocol
In 1987, many countries signed an agreement called the Montreal Protocol. This important treaty aimed to stop making and using CFCs. It was a big step for international cooperation. The Montreal Protocol has been very successful. It has helped the ozone layer slowly start to heal.
What are the alternatives?
Today, we use safer chemicals.
- Hydrofluorocarbons (HFCs): These do not harm the ozone layer. However, some HFCs are still strong greenhouse gases.
- Hydrofluoroolefins (HFOs): These are newer alternatives. They are better for the climate than HFCs.
Scientists continue to work on even safer options. The goal is to protect both the ozone layer and our climate.
Related pages
- Thomas Midgley (American inventor who helped develop CFCs)
- Freon (a brand name for some CFCs and other refrigerants)
Images for kids
-
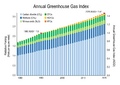
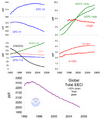
The warming influence of greenhouse gases in the atmosphere has increased substantially in recent years. The rising presence of carbon dioxide from fossil fuel burning is the largest overall driver. The relatively smaller but significant warming impact from releases of the most abundantly produced CFCs (CFC11 and CFC12) will continue to persist for many further decades into the future.
See also
 In Spanish: Clorofluorocarburo para niños
In Spanish: Clorofluorocarburo para niños
 | James B. Knighten |
 | Azellia White |
 | Willa Brown |