History of mass spectrometry facts for kids
The history of mass spectrometry is all about how scientists learned more about tiny particles like atoms and molecules. It started in the mid-1800s when people studied how electricity moved through gases. This led to finding "anode rays" (which are positive ions) and "cathode rays" (which are electrons).
As scientists got better at separating these tiny particles, they discovered stable isotopes. These are like different versions of the same element, but with slightly different weights. For example, neon was found to have two main types: neon-20 and neon-22. Later, special machines called mass spectrometers were even used in the Manhattan Project to separate different types of uranium for making the atomic bomb.


Contents
Early Ideas About Atoms
In the early 1800s, a chemist named William Prout had an interesting idea called Prout's hypothesis. He noticed that the weights of many elements seemed to be simple multiples of the weight of hydrogen. This made him think that all other atoms might just be made up of hydrogen atoms.
However, when scientists measured atomic weights more carefully, they found some elements, like chlorine, didn't fit this idea perfectly. Chlorine's weight was about 35.45 times that of hydrogen, not a whole number. It took almost a hundred years to figure out why this was the case!
Discovering Canal Rays
In the mid-1800s, scientists like Julius Plücker and Johann Wilhelm Hittorf were playing with "discharge tubes." These tubes had gases inside and electricity running through them, making them glow. They saw rays coming from the negative side (the cathode). These were called "cathode rays" and were later found to be streams of electrons.
Then, in 1886, Eugen Goldstein made a special tube with holes in the cathode. He saw new rays passing through these holes, going in the opposite direction from the cathode rays. He called them "Kanalstrahlen," or canal rays. These rays were made of positively charged particles.
Finding Different Types of Atoms: Isotopes
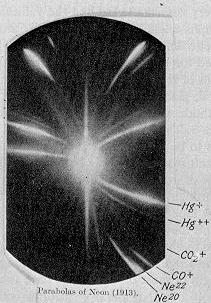
In 1913, a famous scientist named J. J. Thomson was studying these canal rays. He sent a stream of charged neon gas through electric and magnetic fields. When these particles hit a special photographic plate, they left two separate marks. This showed him that neon gas wasn't just one type of atom, but two different types with slightly different weights: neon-20 and neon-22. These different types of the same element are called isotopes.
Thomson's student, Francis William Aston, continued this work. In 1919, he built the first working mass spectrometer. This amazing machine allowed him to find isotopes for many other elements, like chlorine and bromine. He proved that many elements we find in nature are actually mixtures of these different isotopes. Aston's work was so important that he won the Nobel Prize in Chemistry in 1922. He also came up with the "Whole Number Rule," which said that most isotopes have masses very close to whole numbers.
Another scientist, Arthur Jeffrey Dempster, also built a mass spectrometer in 1918. His design is still used today! In 1935, Dempster discovered a very important isotope of uranium, called 235U. This specific isotope was later used to create the atom bomb and nuclear power.
In 1932, Kenneth Bainbridge built an even more precise mass spectrometer. He used it to prove Einstein's famous equation, E = mc2, which shows that mass and energy are related.
Mass Spectrometers and the Manhattan Project
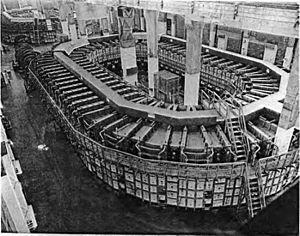
During World War II, scientists needed a way to separate different types of uranium for the Manhattan Project, which was a secret project to build the atomic bomb. Ernest O. Lawrence developed a special type of mass spectrometer called a Calutron. It was named after the University of California where Lawrence worked.
These Calutrons were huge machines used at a plant in Oak Ridge, Tennessee. They helped separate the uranium-235 needed for the "Little Boy" nuclear weapon, which was dropped on Hiroshima in 1945.
Combining Tools: GC-MS
In the 1950s, scientists Roland Gohlke and Fred McLafferty came up with a clever idea: combining a mass spectrometer with another tool called a gas chromatograph. This new tool, called GC-MS, made it much easier to identify different substances in a mixture.
As computers became smaller and cheaper, they made mass spectrometers even easier to use and much faster at analyzing samples.
New Ways to Ionize Molecules
For a long time, it was hard to study large, fragile molecules like proteins using mass spectrometry because the old methods would break them apart. But in the late 1960s and 1980s, new "soft ionization" methods were developed.
- Field Desorption (1969): Developed by Beckey, this method uses a strong electric field to gently pull electrons off molecules, turning them into ions without breaking them.
- Chemical Ionization (1960s): This method uses special "reagent ions" to gently transfer a charge to the sample molecules. It's less harsh than older methods, so the molecules stay mostly whole.
- Electrospray Ionization (1984): John Bennett Fenn and his team found a way to spray a liquid sample into tiny charged droplets. As the liquid evaporates, the molecules become charged ions. This was a huge step for studying large biological molecules. Fenn shared a Nobel Prize for this work in 2002.
- Matrix-Assisted Laser Desorption/Ionization (MALDI) (1985): Franz Hillenkamp and Michael Karas discovered that if you mix a large molecule with a special "matrix" substance and then hit it with a laser, the matrix absorbs the laser energy and helps the large molecule become an ion without breaking apart. In 1987, Koichi Tanaka used a similar method to ionize very large proteins. Tanaka also shared the Nobel Prize in 2002 for this breakthrough.
These new methods revolutionized how scientists could study complex biological molecules like proteins, which are essential for life.

Important Moments in Mass Spectrometry
19th Century Discoveries
- 1886: Eugen Goldstein sees canal rays (positive rays).
- 1898: Wilhelm Wien shows that canal rays can be bent by electric and magnetic fields, and that their particles are much heavier than electrons.
- 1898: J. J. Thomson measures the mass-to-charge ratio of electrons.
20th Century Breakthroughs
- 1913: J. J. Thomson separates the 20Ne and 22Ne isotopes of neon.
- 1919: Francis Aston builds the first working mass spectrograph.
- 1922: Aston wins the Nobel Prize for discovering isotopes.
- 1931: Ernest O. Lawrence invents the cyclotron, a particle accelerator.
- 1942: Lawrence develops the Calutron to separate uranium isotopes for the Manhattan Project.
- 1953: Wolfgang Paul and Helmut Steinwedel introduce the quadrupole mass filter, a new way to sort ions.
- 1959: Scientists connect a gas chromatograph to a mass spectrometer for the first time.
- 1966: F. H. Field and M. S. B. Munson develop chemical ionization.
- 1984: John Bennett Fenn uses electrospray to ionize large biomolecules.
- 1985: Franz Hillenkamp and Michael Karas describe and name matrix-assisted laser desorption ionization (MALDI).
- 1987: Koichi Tanaka uses a special method to ionize whole proteins.
- 1989: Wolfgang Paul wins the Nobel Prize for developing the ion trap technique.
21st Century Developments
- 2002: John Bennett Fenn and Koichi Tanaka share a Nobel Prize for their work on soft ionization methods, which made it possible to analyze large biological molecules.
- 2005: The Orbitrap mass spectrometer becomes available for commercial use, offering very high precision.
See also
 | Toni Morrison |
 | Barack Obama |
 | Martin Luther King Jr. |
 | Ralph Bunche |