Chirality facts for kids
Chirality is a cool science word for when an object or a molecule exists in two forms that are mirror images of each other. Think about your hands! Your left hand is a mirror image of your right hand. Try to put your left hand exactly on top of your right hand so they match perfectly – you can't, no matter how you turn them. This is why you can't wear a left-handed glove on your right hand!
Contents
What is Chirality in Chemistry?
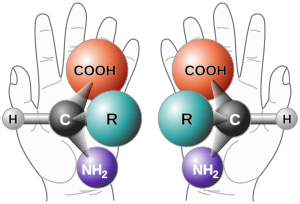
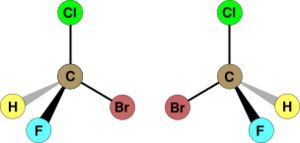
In chemistry, chirality is super important when we talk about molecules. If a molecule is chiral, it means it has two forms that are mirror images of each other. These two forms are exactly the same in every other way, but they just can't be placed perfectly on top of each other.
Chemists call these mirror-image molecules enantiomers. Sometimes they are also called optical isomers. Because the difference between right and left hands is easy to understand, chemists often call enantiomers either 'right-handed' or 'left-handed'.
Racemic Mixtures
If you have a mix of a chiral molecule where there are equal amounts of the 'right-handed' and 'left-handed' forms, it's called a racemic mixture.
Chirality in molecules is very common. You can find it in many different areas of chemistry, like stereochemistry, inorganic chemistry, organic chemistry, physical chemistry, and biochemistry.
The way a molecule is shaped, or its symmetry, helps us know if it's chiral. A molecule is achiral (meaning not chiral) if it has a special line or plane of symmetry that makes it look the same on both sides.
Chirality in Biology
Many important molecules in living things are chiral. This includes amino acids, which are the tiny building blocks that make up proteins, and also sugars. What's really interesting is that most of these biological molecules have the same chirality. For example, most amino acids are 'L' (left-handed), and most sugars are 'D' (right-handed).
Scientists have a lot of discussions about why living things mostly use one type of chirality. This idea is called homochirality. Many believe it happened by chance a long, long time ago when life first started. They think that if there's life elsewhere in the universe, it might use molecules with different chiralities!
Enzymes are special proteins that help chemical reactions happen in our bodies. Enzymes are also chiral. They usually only work with one specific mirror-image form of a molecule. Imagine an enzyme as having a glove-like pocket. If this 'glove' is shaped for a 'right-handed' molecule, then only that specific molecule will fit inside and work with the enzyme. The 'left-handed' version won't fit well and won't react.
It was very efficient for living things to choose one form of each chiral compound. This way, our bodies don't need two different enzymes for every single chemical reaction that involves chiral molecules!
Images for kids
See also
 In Spanish: Quiralidad para niños
In Spanish: Quiralidad para niños