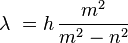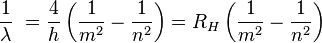Johann Jakob Balmer facts for kids
Quick facts for kids
Johann Jakob Balmer
|
|
|---|---|
 |
|
| Born | 1 May 1825 |
| Died | 12 March 1898 (aged 72) Basel, Switzerland
|
| Nationality | Swiss |
| Alma mater | University of Basel |
| Scientific career | |
| Fields | Mathematics |
Johann Jakob Balmer (born May 1, 1825 – died March 12, 1898) was a Swiss mathematician. He is most famous for his work in physics. He discovered a special formula for the Balmer series of the hydrogen atom.
About Johann Jakob Balmer
Johann Jakob Balmer was born in Lausen, Switzerland. His father, also named Johann Jakob Balmer, was a chief judge. Johann was the oldest son in his family.
He was very good at mathematics in school. So, he decided to study math at university. He went to the University of Karlsruhe and the University of Berlin. In 1849, he earned his PhD from the University of Basel. His special paper for his PhD was about the cycloid.
Johann Balmer spent his whole life in Basel. He taught at a school for girls there. He also gave lectures at the University of Basel. In 1868, when he was 43, he married Christine Pauline Rinck. They had six children together.
Balmer's Big Discovery
Even though Balmer was a mathematician, he is best known for his work on something called "spectral series." In 1885, when he was 60 years old, he made a very important discovery. He found a formula for the visible spectral lines of the hydrogen atom.
His friend, Eduard Hagenbach-Bischoff, suggested he study these lines. Balmer used measurements of hydrogen lines made by Anders Jonas Ångström. He created a formula to figure out the wavelength of these lines:
In this formula, n is 2. The letter m can be 3, 4, 5, 6, and so on. The letter h is a special number, 3.6456 · 10−7 meters, or 364.56 nanometers. Balmer called h the "fundamental number of hydrogen." Today, we call it the Balmer constant.
Balmer used his formula to guess the wavelength for m = 7:
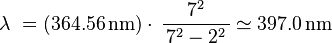
Hagenbach told Balmer that Ångström had already seen a line with a wavelength of 397 nm. This part of the hydrogen light spectrum is now known as the Balmer series. It comes from electrons moving between energy levels.
The Balmer lines are specific colors of light that hydrogen gives off. You can see them in the visible light spectrum. These lines are at 410.29 nm, 434.17 nm, 486.27 nm, and 656.47 nm. They happen when electrons in an excited state (meaning they have extra energy) release a tiny packet of light called a photon. Then, they drop back to a lower energy level (n = 2) in the hydrogen atom.
Two of Balmer's friends, Hermann Wilhelm Vogel and William Huggins, later found other Balmer series lines. They saw them in the light from white stars.
Balmer's formula was later found to be a special part of the Rydberg formula. This formula was created by Johannes Rydberg in 1888.
Here, 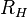 is the Rydberg constant for hydrogen. For Balmer's formula,
is the Rydberg constant for hydrogen. For Balmer's formula, 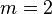 and
and 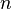 is always greater than
is always greater than 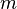 .
.
People didn't fully understand why these formulas worked until after Balmer died. In 1913, Niels Bohr introduced his Bohr model of the atom. This model helped explain how electrons move in atoms and why they produce specific light lines.
Johann Balmer passed away in Basel when he was 72 years old.
Things Named After Balmer
- The Balmer formula and the Balmer constant h are named after him.
- The Balmer lines and Balmer series are also named in his honor.
- The Balmer jump is a term used in Astronomy. It helps scientists classify different types of stars.
- A crater on the Moon is called Balmer after him.
- A minor planet (a small object orbiting the Sun) called 12755 Balmer is also named after him.
See also
 In Spanish: Johann Jakob Balmer para niños
In Spanish: Johann Jakob Balmer para niños
 | Ernest Everett Just |
 | Mary Jackson |
 | Emmett Chappelle |
 | Marie Maynard Daly |