Leclanché cell facts for kids
The Leclanché cell is a type of battery that was invented by a French scientist named Georges Leclanché in 1866. This battery used a special liquid called an electrolyte, which was made of ammonium chloride. It also had a positive part called a cathode made of carbon, a chemical called manganese dioxide to help it work, and a negative part called an anode made of zinc. The ideas behind this battery were later used to create the dry cell batteries we use today.
Contents
How It Was Invented
In 1866, Georges Leclanché created his new battery. It had a zinc rod as the negative part and a manganese dioxide rod, mixed with a bit of carbon, as the positive part. These parts were placed in a jar filled with ammonium chloride solution. The carbon helped the battery conduct electricity better. This battery produced about 1.4 volts of power. It quickly became very popular for things like telegraphy, signals, and electric bells.
Early dry cell versions of the Leclanché battery were used to power old telephones. These batteries were usually kept in a wooden box on the wall. The Leclanché cell wasn't great for long conversations because it couldn't provide a steady flow of electricity for very long. If you talked too much, the battery would get weak, and you couldn't hear the conversation anymore. This happened because some chemical reactions inside the battery would make it harder for electricity to flow. However, if you let the battery rest, it would recover. This made it good for short uses with breaks in between, but not for long, continuous use.
How It Was Built
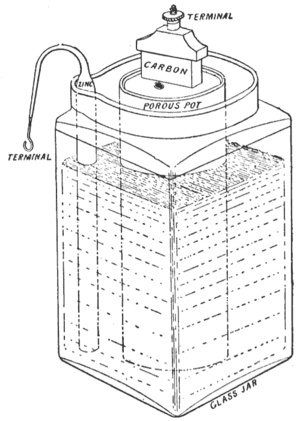
The first Leclanché cell used a special "porous pot" inside. This pot made it a bit harder for electricity to flow easily. Over time, people made changes to improve it and make it work better. Both Leclanché and later Carl Gassner worked to turn this "wet" battery into a more useful and portable dry cell.
Different Types of Cells
Porous Pot Cell
In Leclanché's first design, crushed manganese dioxide was packed into a pot. A carbon rod was put into this pot to act as the positive part (cathode). The negative part (anode) was a zinc rod. Both the pot and the zinc rod were placed in a liquid solution of ammonium chloride. This liquid, called the electrolyte, soaked through the porous pot to reach the carbon rod.
Agglomerate Block Cell
In 1871, Leclanché changed his design. He got rid of the porous pot. Instead, he used two "agglomerate blocks" that were attached to the carbon plate with rubber bands. These blocks were made by mixing manganese dioxide with other materials and pressing them into shapes.
Sack Cell
This version replaced the porous pot with a wrapping made of canvas or a similar material. Also, the zinc rod was changed to a zinc cylinder. This larger surface area helped the battery work better and made it easier for electricity to flow.
Adding Starch
In 1876, Georges Leclanché tried adding starch to the ammonium chloride liquid. This made the liquid thicker, like a gel, which was a step towards making a dry cell.
Improved Dry Cell
In 1888, a German doctor named Carl Gassner made the gelling process even better. He created a more portable dry cell by mixing plaster and other water-absorbing chemicals with the ammonium chloride electrolyte.
How It Makes Electricity
The Leclanché cell creates electricity through a chemical process. Here's a simpler way to think about it:
1. At the Zinc (Negative) Part: Zinc atoms on the surface of the negative part (anode) give up their electrons. When they lose electrons, they become positively charged ions. These electrons are left behind on the zinc, making it more negatively charged. 2. Electron Flow: When you connect the battery to something that needs power, these extra electrons on the zinc flow through the wire to the carbon rod (positive part). This flow of electrons is what we call electric current. 3. At the Carbon (Positive) Part: When the electrons reach the carbon rod, they combine with manganese dioxide and water. This reaction creates new substances and negatively charged hydroxide ions. 4. Inside the Liquid: The hydroxide ions then react with the ammonium ions in the ammonium chloride liquid. This creates ammonia gas and water.
All these chemical changes work together to keep the electrons flowing and produce electricity. The Leclanché cell typically produced about 1.4 volts of power.
What It Was Used For
The Leclanché cell produced about 1.4 volts of power. It was widely used in telegraphy, signals, electric bells, and other devices that needed power only sometimes. It was good for these uses because it didn't need much looking after.
The "wet" Leclanché battery was the first step towards the modern zinc–carbon battery, which is a dry cell. Later, people found that adding zinc chloride to the battery's paste could increase its power to 1.5 volts. Even later, the ammonium chloride was removed completely, leading to batteries that could last longer without losing power as quickly.
See also
 In Spanish: Pila Leclanché para niños
In Spanish: Pila Leclanché para niños
- List of battery types
- History of the battery
- Alkaline battery, a similar battery where a different chemical (KOH) was used instead of ammonium chloride. This improved battery could store much more power and became popular later (around the 1960s and 1970s).
 | James B. Knighten |
 | Azellia White |
 | Willa Brown |