Molecular physics facts for kids
Molecular physics is a science that studies molecules. It looks at how molecules are built and how they move. This field is a lot like physical chemistry and quantum chemistry. It is often seen as a part of atomic, molecular, and optical physics.
Scientists in molecular physics study how molecules are put together. They also look at how tiny parts inside molecules behave. This science uses ideas from both older physics and modern quantum physics. It helps us understand how light and other energy waves interact with matter. Experiments often use tools like spectroscopy, which studies how light interacts with matter.
Contents
How Molecules Are Built
Inside a molecule, tiny electrons and nuclei (the centers of atoms) pull on each other. This pulling is called the Coulomb interaction. Even though the forces are similar, the nuclei stay mostly in place. The electrons, however, move around a lot more. This happens because nuclei are much heavier than electrons. So, electrons move more easily when the same force acts on them.
Molecular Energy Levels and Light
When atoms join to form molecules, their inner electrons stay close to their original atom's center. But the outer electrons, called valence electrons, spread out around the whole molecule. How these outer electrons are arranged sets the molecule's electronic energy level. This idea is similar to how atomic orbital theory describes single atoms.
Imagine energy levels like steps on a ladder. Electrons can only be on certain steps. The energy difference between these steps for electrons is quite large. This means that when electrons change steps, they often absorb or give off light that we can see, or even ultraviolet light.
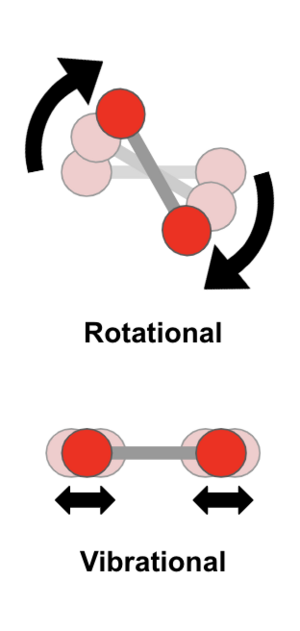
Molecules also have other special energy levels. These are for how they vibrate and spin.
- Vibrational energy levels are about the nuclei shaking back and forth. Think of the atoms in a molecule as being connected by tiny springs. When they vibrate, they shake. The energy differences for these vibrations are smaller than for electrons. This means they absorb or give off infrared light.
- Rotational energy states describe the whole molecule spinning around. These energy differences are the smallest. They involve light in the far infrared and microwave regions.
Sometimes, molecules can change their electronic, vibrational, and rotational states all at once. For example, a "rovibrational" change means both spinning and shaking change. These combined changes can involve many different types of light.
Experiments with Molecules
Scientists do experiments to learn about molecules. They want to find out:
- Their shape and size.
- Their electric and magnetic properties.
- Their internal energy levels.
- How much energy it takes to ionize (remove an electron) or dissociate (break apart) them.
For shape and size, scientists use rotational and vibrational light patterns. These patterns help them figure out how far apart the atoms are in a molecule. X-ray diffraction is another way to measure these distances, especially for molecules with heavy atoms. All types of spectroscopy help scientists map out the energy levels of molecules.
New Discoveries
Today, scientists are using molecules in many exciting ways. They use them to check if the basic rules of physics are correct. Some molecules are very sensitive to tiny changes that could point to new physics.
Molecules might also be used in the future for quantum computers. Their complex energy levels could help store information more efficiently than single atoms. Also, scientists study how energy moves around inside a molecule after it gets excited. This helps them understand how molecules react and change.
See also
 In Spanish: Física molecular para niños
In Spanish: Física molecular para niños
- Born–Oppenheimer approximation
- Molecular energy state
- Molecular modeling
- Rigid rotor
- Spectroscopy
- Physical chemistry
- Chemical Physics
- Quantum Chemistry
 | Toni Morrison |
 | Barack Obama |
 | Martin Luther King Jr. |
 | Ralph Bunche |