Noble gas compound facts for kids
Noble gas compounds are special chemical compounds. They contain an element from Group 18 of the periodic table. Group 18 is where all the noble gases are found. For a long time, scientists thought these gases could not form compounds. But now we know they can!
Discovering Noble Gas Compounds
For many years, scientists believed that noble gases could not form any chemical compounds. This was because their outer electron shells were already full. This meant they couldn't easily gain or lose electrons, which is usually needed for bonding.
However, in 1933, a famous scientist named Linus Pauling had a different idea. He predicted that heavier noble gases, like xenon, could actually react with very reactive elements such as fluorine or oxygen. These elements are very "electronegative," meaning they strongly attract electrons.
Pauling's prediction turned out to be correct! Later, xenon hexafluoride (XeF6) was discovered. Since then, many more noble gas compounds have been found, showing that these "noble" gases are not always so unreactive after all.
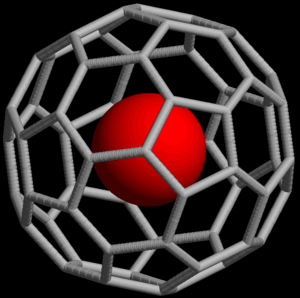
Noble Gases Inside Fullerenes
Noble gases can also form unique compounds called endohedral fullerenes. Imagine a tiny cage made of carbon atoms; that's a fullerene molecule. In an endohedral fullerene, a noble gas atom gets trapped inside this carbon cage!
In 1993, scientists found a way to make these compounds. They put C60 fullerenes (which have 60 carbon atoms) under pressure with helium or neon gas. This created He@C60 and Ne@C60, where the noble gas atom is inside the fullerene.
At first, it was hard to get many of these. Only about one out of every 650,000 C60 molecules would trap a helium atom at low pressure. But by using much higher pressures (around 3000 bar), they could get up to 0.1% of the fullerenes to contain a noble gas atom.
Scientists have also made endohedral fullerenes with argon, krypton, and xenon. These special compounds help us learn more about how atoms interact even when they are trapped inside other molecules.
Images for kids
-
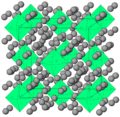
Kr(H2)4 and H2 solids formed in a diamond anvil cell. Ruby was added for pressure measurement.
See also
In Spanish: Compuesto de gas noble para niños