Atomic orbital facts for kids
Atomic orbitals are like special areas around the center of an atom where you're most likely to find electrons. Think of them as "electron homes" within an atom. These areas are described by a special math rule that shows how electrons move in a wavy way.
The word 'orbital' might make you think of planets orbiting the sun. Early scientists thought electrons orbited the nucleus in a similar way. But now we know electrons move in more complex, wave-like patterns.
The number of atomic orbitals in a chemical element depends on its place in the periodic table. Electrons can move between these orbitals. Where they go depends on their energy and how many other electrons are around.
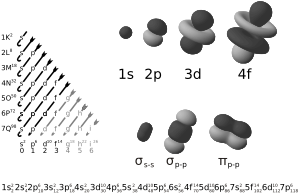
In atomic theory, an atomic orbital is a special number that describes an electron's state. Each orbital can hold one or two electrons. How these orbitals are arranged helps us understand the electron configuration of atoms. The names of orbitals (like s, p, d, f) came from how early scientists described certain light patterns they saw from alkali metals. These patterns were called sharp, principal, diffuse, and fundamental.
Contents
What are Orbitals?
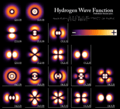
Atomic orbitals are not fixed paths like planets orbiting the sun. Instead, they are regions where an electron is most likely to be found. Imagine a fuzzy cloud around the nucleus; the denser parts of the cloud are where the electron spends more time.
How Electrons Fill Orbitals
Electrons fill orbitals in a specific order, usually starting with the lowest energy levels. Each orbital can hold a maximum of two electrons. This arrangement helps determine how atoms interact with each other to form bonds.
Different Shapes of Orbitals
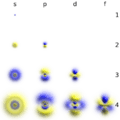
Orbitals come in different shapes and sizes.
- s-orbitals are shaped like a sphere.
- p-orbitals look like two balloons tied together at the nucleus.
- d-orbitals have more complex shapes, often like four-leaf clovers or two balloons with a donut around the middle.
- f-orbitals are even more complicated in their shapes.
These shapes are important because they affect how atoms bond together.
Related pages
Images for kids
See also
 In Spanish: Orbital atómico para niños
In Spanish: Orbital atómico para niños