Ryōji Noyori facts for kids
Quick facts for kids
Ryōji Noyori
|
|
|---|---|

Noyori in 2002
|
|
| Born | September 3, 1938 |
| Nationality | Japanese |
| Alma mater | Kyoto University |
| Awards |
|
| Scientific career | |
| Fields |
|
| Institutions | |
| Doctoral advisor | Hitoshi Nozaki |
| Other academic advisors | Elias J. Corey |
Ryōji Noyori (野依 良治, Noyori Ryōji, born September 3, 1938) is a famous Japanese chemist. He won the Nobel Prize in Chemistry in 2001. He shared half of the prize with William S. Knowles. They were recognized for their work on "chirally catalyzed hydrogenations." This is a special way to make molecules that are mirror images of each other. The other half of the prize went to K. Barry Sharpless for similar work.
Contents
Early Life and Education
Ryōji Noyori was born in Kobe, Japan. When he was young, he was very interested in physics. This interest grew because of Hideki Yukawa, a famous physicist and a friend of his father. Yukawa won the Nobel Prize in Physics in 1949.
Later, Ryōji Noyori became fascinated with chemistry. He heard a presentation about nylon at an industrial fair. He realized that chemistry could "produce high value from almost nothing." This meant making useful things from simple materials.
University Studies
Noyori studied at Kyoto University in Japan. He graduated in 1961 with a degree in Industrial Chemistry. He then earned a Master's degree from the same university. From 1963 to 1967, he worked as a research associate at Kyoto University. He also taught in the research group of Hitoshi Nozaki.
In 1967, Noyori earned his Doctor of Engineering degree from Kyoto University. He became an associate professor there in 1968. He also did research at Harvard University in the United States. In 1972, he became a full professor at Nagoya University in Japan. He has been based at Nagoya University ever since. From 2003 to 2015, he was the president of RIKEN. RIKEN is a very large national research organization in Japan.
Important Discoveries in Chemistry



Ryōji Noyori strongly believes in the power of catalysis. A catalyst is something that speeds up a chemical reaction without being used up itself. He also believes in green chemistry. This means designing chemical products and processes that reduce harm to the environment.
He once said that making simple and practical chemical processes is "indispensable to the survival of our species." He also believes that "Research is for nations and mankind, not for researchers themselves." He encourages scientists to help shape public opinion and government policies. This is to help build a "sustainable society" for the future.
Asymmetric Hydrogenation
Noyori is most famous for a process called asymmetric hydrogenation. This process uses special catalysts made from metals like rhodium and ruthenium. These catalysts help create molecules that have a specific "handedness." Think of your left and right hands; they are mirror images but not identical. Many molecules in nature also have this "handedness."
His method is used to make important medicines. For example, it helps produce naproxen. This is a common medicine used to reduce pain and swelling. It also helps make levofloxacin, which is an antibacterial medicine.
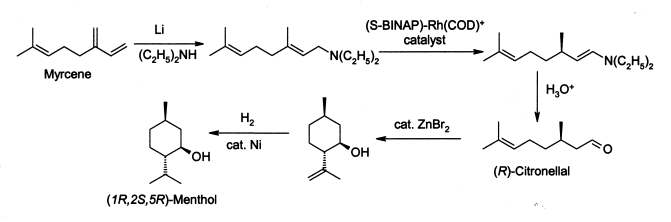
Making Menthol
Noyori's methods are also used to make menthol. Menthol is what gives mint its cool feeling and smell. Each year, thousands of tons of menthol are produced using his special method. This process makes menthol with a very high purity.
More recently, Noyori worked with Philip G. Jessop. They developed a way to make N,N-dimethylformamide. This is a common chemical used in many industries. They made it from hydrogen, dimethylamine, and carbon dioxide. They used a special catalyst in a "supercritical fluid" state.
Awards and Honors
Ryōji Noyori has received many awards for his important work. The The Ryoji Noyori Prize is named in his honor. He has also received honorary doctorates from several universities around the world. In 2005, he became a Foreign Member of the Royal Society in the UK.
Here are some of his major awards:
- 1992 – Asahi Prize
- 1993 – Tetrahedron Prize
- 1997 – Arthur C. Cope Award
- 2001 – Wolf Prize in Chemistry
- 2001 – Nobel Prize for Chemistry
- 2009 – Lomonosov Gold Medal
See also
 In Spanish: Ryōji Noyori para niños
In Spanish: Ryōji Noyori para niños
- List of Japanese Nobel laureates
- List of Nobel laureates affiliated with Kyoto University
 | Janet Taylor Pickett |
 | Synthia Saint James |
 | Howardena Pindell |
 | Faith Ringgold |


