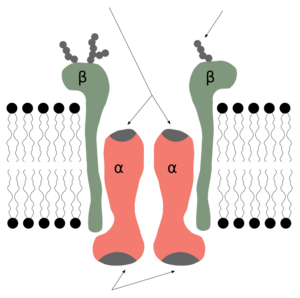Sodium–potassium pump facts for kids
Quick facts for kids Na⁺/K⁺-ATPase pump |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
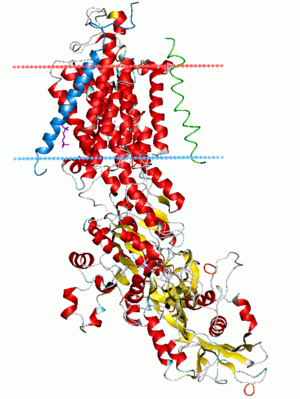
Sodium–potassium pump, E2-Pi state. Calculated hydrocarbon boundaries of the lipid bilayer are shown as blue (intracellular) and red (extracellular) planes
|
|||||||||
| Identifiers | |||||||||
| EC no. | 7.2.2.13 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
|
|||||||||
The sodium–potassium pump (also called Na⁺/K⁺-ATPase) is a special enzyme found in the outer layer (the membrane) of all animal cells. Think of it as a tiny machine that works like a pump. It helps cells do many important jobs.
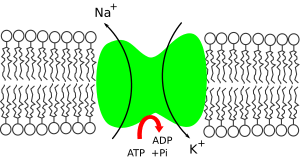
This pump is "active," meaning it uses energy from a molecule called ATP. For every ATP molecule it uses, the pump moves three sodium ions (Na⁺) out of the cell and brings two potassium ions (K⁺) into the cell. This means it sends out one more positive charge than it brings in. This action makes the amount of sodium outside the cell much higher than inside, and the amount of potassium inside the cell much higher than outside.
A Danish scientist named Jens Christian Skou discovered the sodium–potassium pump in 1957. He won a Nobel Prize in 1997 for this important discovery. His work helped us understand how cells control what goes in and out. This is super important for cells that send signals, like nerve cells. They rely on this pump to react to things and send messages.
Humans and other mammals have four slightly different types of sodium pumps. Each type works best in different parts of the body.
Contents
How the Pump Works
The Na⁺/K⁺-ATPase pump does many things for cells. It helps keep the cell's electrical balance, moves important substances, and controls the cell's size. It also helps cells respond to signals. Cells use a lot of their energy (sometimes up to 70% in nerve cells!) just to keep the right amounts of sodium and potassium inside.
For nerve cells, this pump can use up to three-quarters of the cell's energy! This energy often comes from a process called glycolysis, which breaks down sugar for power.
Keeping Cells Electrically Balanced
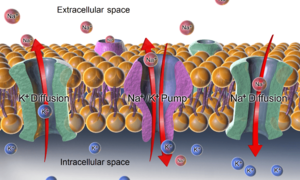
To keep the cell working correctly, cells need to have low amounts of sodium ions and high amounts of potassium ions inside. The sodium–potassium pump helps with this. It moves 3 sodium ions out and 2 potassium ions in. This creates an electrical difference across the cell membrane, which is called the resting potential. This electrical difference is key for nerve and muscle cells to work.
Moving Substances
The pump also helps move other important things into the cell. When sodium ions are pumped out, it creates a "gradient" (a difference in concentration). Other special proteins then use this sodium gradient like a driving force. For example, they can bring glucose (sugar) and amino acids (building blocks for proteins) into the cell.
In your gut, the pump helps move sodium out of cells. Then, a "symporter" protein uses this sodium difference to bring both sodium and glucose into the cell. This is much more efficient than just letting things drift in. Similar processes happen in your kidneys.
Controlling Cell Size
If the Na⁺-K⁺ pumps stop working, cells can swell up. This is because of something called osmosis. Cells have many ions and proteins inside, which makes the inside "saltier" than the outside. Water naturally flows into the cell to balance this out. If too much water enters, the cell can burst! The Na⁺-K⁺ pump helps keep the right balance of ions, which stops too much water from coming in and keeps the cell at the right size.
If a cell starts to swell, the pump automatically becomes more active. This helps it quickly fix the ion balance and prevent damage.
Sending Signals
Scientists have found that this pump does more than just move ions. It can also send signals inside the cell. For example, it can help control how cells grow and respond to their environment. It works with other proteins to create a signaling team.
One example is how the pump interacts with a protein called Src. When a certain molecule (called ouabain) attaches to the pump, it can activate Src. This starts a chain of events that sends messages throughout the cell.
Controlling Brain Activity
The Na⁺-K⁺ pump is very important for how nerve cells in the brain work. It helps control the activity of specific nerve cells, like those in the cerebellum (which helps with balance and coordination). This suggests the pump isn't just a "housekeeping" tool for cells, but also plays a role in how the brain thinks and processes information.
Problems with the Na⁺-K⁺ pump can lead to brain issues, like a movement disorder called dystonia-parkinsonism or even epilepsy. Alcohol can also affect these pumps in the cerebellum, which is why drinking too much can mess with your coordination.
How the Pump Works Step-by-Step
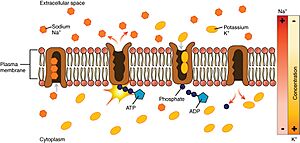
Let's look at how the pump works, starting from inside the cell:
- The pump prefers to grab onto sodium ions (Na⁺) more than potassium ions (K⁺). So, after it gets energy from ATP, it picks up 3 sodium ions from inside the cell.
- The ATP molecule is then broken down, which gives energy to the pump. This causes the pump to change its shape.
- This change in shape moves the sodium ions to the outside of the cell. Now, the pump doesn't like sodium ions anymore, so it lets them go. Instead, it really likes potassium ions.
- The pump then grabs 2 potassium ions from outside the cell. This makes the pump change back to its original shape, releasing the potassium ions into the cell.
- Once the potassium ions are released, the pump is ready to pick up sodium ions again. A new ATP molecule attaches, and the whole process starts over!
How the Pump is Controlled
Inside the Body
The Na⁺/K⁺-ATPase pump can be made more active by certain signals inside the body, like a molecule called cAMP. If something increases cAMP, the pump works harder. If something decreases cAMP, the pump slows down.
The pump's activity can also be changed by adding or removing small chemical groups (like phosphate) in a process called reversible phosphorylation. For example, in animals that go into a sleep-like state called estivation, the pump becomes less active.
Outside the Body (Medicines)
We can also control the Na⁺/K⁺-ATPase pump using medicines. For example, thyroid hormones can change how much of the pump is made in cells.
Medicines called cardiac glycosides (like digoxin) are used to treat heart problems. They work by affecting the Na⁺/K⁺-ATPase pump in heart cells. When these medicines slow down the pump, it causes more calcium to build up inside the heart muscle cells. This extra calcium makes the heart muscle contract more strongly, which can help a weak heart pump blood better.
Discovery of the Pump
Jens Christian Skou discovered the Na⁺/K⁺-ATPase in 1957. He was working as a professor at the University of Aarhus in Denmark. He published his findings that same year.
In 1997, he was awarded half of the Nobel Prize in Chemistry. He received it "for the first discovery of an ion-transporting enzyme, Na⁺,K⁺-ATPase."
Genes for the Pump
The instructions for making the different parts of the Na⁺/K⁺-ATPase pump are found in our genes. There are different genes for the "alpha" parts and the "beta" parts of the pump.
- Alpha genes: ATP1A1, ATP1A2, ATP1A3, ATP1A4.
- Beta genes: ATP1B1, ATP1B2, ATP1B3, ATP1B4.
The Pump in Insects
Scientists have studied the Na⁺/K⁺-ATPase pump in insects. They found that some insects that eat plants containing certain toxic chemicals (called cardenolides) have special changes in their pumps. These changes help the insects resist the poisons. This shows how animals can adapt to their environment!
See also
 In Spanish: Bomba sodio-potasio para niños
In Spanish: Bomba sodio-potasio para niños
- Thyroid hormone
- V-ATPase
Images for kids
-
Sodium–potassium pump, E2-Pi state. Calculated hydrocarbon boundaries of the lipid bilayer are shown as blue (intracellular) and red (extracellular) planes
-
The Na⁺/K⁺-ATPase, as well as effects of diffusion of the involved ions maintain the resting potential across the membranes.
-
The sodium–potassium pump is found in many cell (plasma) membranes. Powered by ATP, the pump moves sodium and potassium ions in opposite directions, each against its concentration gradient. In a single cycle of the pump, three sodium ions are extruded from and two potassium ions are imported into the cell.