State of matter facts for kids
Imagine all the different forms things can take! In physics, a state of matter is how matter exists. It's like different outfits for the same stuff. You see four main states every day: solid, liquid, gas, and plasma.
These states are different because of how their tiny parts (like atoms and molecules) are arranged. Also, how these parts move together changes.
- In a solid, particles are packed tight. They stay in fixed spots, just vibrating. This gives solids a clear shape and size.
- In a liquid, particles are still close. But they can slide past each other. This means liquids keep their size but take the shape of their container.
- In a gas, particles are far apart. They move around freely. Gases spread out to fill any container and take its shape.
- Plasma is like a gas, but it has charged particles. These particles (called ions and electrons) move on their own. They react to electric and magnetic forces.
Besides these four, many other states of matter exist. Some are in-between, like liquid crystals. They act a bit like solids and a bit like liquids. Other states appear under very special conditions. These include super-cold states or those found in stars.
Contents
The Four Main States of Matter
Solids: Keeping Their Shape
In a solid, tiny particles (like ions, atoms, or molecules) are packed very close. The forces between these particles are strong. This means particles cannot move freely. They can only vibrate in their fixed spots.
Because of this, a solid has a stable, definite shape. It also has a definite volume (size). Solids only change their shape if an outside force breaks or cuts them.
Some solids are crystalline. Their particles are arranged in a regular, repeating pattern. For example, iron has different crystal structures at different temperatures. Ice (solid water) has many known crystal structures. These depend on temperature and pressure.
Other solids, like glass, are called amorphous solids. They don't have a long-range, ordered pattern. They are more like a "frozen" liquid.
Solids can turn into liquids by melting. Liquids can turn into solids by freezing. Solids can also turn directly into gases through sublimation. Gases can turn directly into solids through deposition.
Liquids: Taking the Container's Shape
A liquid is a fluid that takes the shape of its container. But it keeps a nearly constant volume. This volume stays the same even if the pressure changes.
When a solid is heated enough, it melts and becomes a liquid. The forces between liquid particles are still important. But the particles have enough energy to move past each other. This makes the liquid flow.
So, a liquid does not have a definite shape. Its shape is set by its container. The volume of a liquid is usually bigger than its solid form. Water is a famous exception; ice takes up more space than liquid water.
Gases: Filling Any Space
A gas is a fluid that can be compressed (squeezed). A gas not only takes the shape of its container. It also expands to fill the entire container.
In a gas, molecules have a lot of kinetic energy (energy of motion). The forces between molecules are very small. The distance between molecules is much larger than the molecules themselves.
A gas has no definite shape or volume. It fills the whole container it is in. You can turn a liquid into a gas by heating it to its boiling point. You can also reduce the pressure at a constant temperature.
Below a certain temperature (its critical temperature), a gas is also called a vapor. A vapor can turn into a liquid just by being compressed. A vapor can exist with a liquid (or solid) at the same time.
A supercritical fluid is a gas under very high temperature and pressure. In this state, you can't tell the difference between a liquid and a gas. These fluids have special uses. For example, supercritical carbon dioxide helps remove caffeine from coffee.
Plasma: The Super-Hot State

Plasma is often made by heating a gas to very high temperatures. This causes electrons to leave their atoms. This creates free electrons and charged atoms (ions). This mix of charged particles is plasma.
You can also make plasma with a huge voltage difference. This is how lightning works.
Plasma is not common on Earth under normal conditions. But it is often created in electric sparks and fluorescent lights. The Sun's corona and stars are all made of plasma. In fact, 99% of all ordinary matter in the universe is plasma!
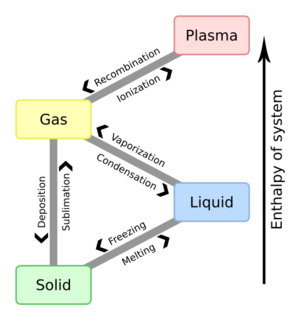
How States Change: Phase Transitions
A state of matter can change into another. These changes are called phase transitions. A phase transition means a change in how the particles are arranged. You can see this by a sudden change in properties.
For example, water can be solid (ice), liquid (water), or gas (steam). Each is a different state. But even ice can have different solid forms. These depend on pressure and temperature.
When you heat a substance, it changes state. Near absolute zero (the coldest possible temperature), most things are solid. As you add heat, a solid melts into a liquid. If you add more heat, it boils into a gas. If you heat it even more, it can become a plasma. In plasma, electrons get so much energy they leave their atoms.
|
To
From
|
Solid | Liquid | Gas | Plasma |
|---|---|---|---|---|
| Solid | Melting | Sublimation | ||
| Liquid | Freezing | Vaporization | ||
| Gas | Deposition | Condensation | Ionization | |
| Plasma | Recombination |
Sometimes, changes happen in steps. These in-between steps are called mesophases. Liquid crystals are a good example of these phases. They are used in liquid crystal displays (LCDs).
Beyond the Basics: Other States of Matter
Glass: A Frozen Liquid?
Glass is a special kind of solid. It is non-crystalline, meaning its particles are not arranged in a perfect, repeating pattern. Think of it as a liquid that has been frozen so quickly that its particles didn't have time to line up.
When glass is heated, it doesn't melt sharply like ice. Instead, it slowly becomes softer and more liquid-like. This change is called a glass transition. Glass can be made from many materials, like the silicates in window glass.
Liquid Crystals: Both Liquid and Solid
Liquid crystals are fascinating states of matter. They have properties that are in-between liquids and solids. They can flow like a liquid. But their molecules also show some order, like in a solid.
For example, in a nematic phase, long, rod-like molecules can flow. But they all tend to point in the same direction. This order makes them react to polarized light. This is why they are used in liquid crystal displays (LCDs) for screens.
Magnets and Their States
Some materials have tiny magnetic moments. These come from the spin of electrons in their atoms. In certain solids, these magnetic moments can line up in special ways. This creates different magnetic states.
In a ferromagnet, like solid iron, all the magnetic moments point in the same direction. This makes the material a strong magnet. If these tiny magnetic areas (domains) also line up, it becomes a permanent magnet. Heating a magnet to its Curie point makes it lose its magnetism. For iron, this happens at 768 degrees Celsius (1414 degrees Fahrenheit).
An antiferromagnet has magnetic moments that point in opposite directions. They cancel each other out. So, the material has no overall magnetism.
A ferrimagnet also has opposite magnetic moments. But they are not equal. This means there is still some overall magnetism. Magnetite is an example of a ferrimagnet.
Super-Cold States: Bose-Einstein Condensates
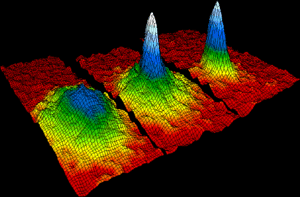
Imagine matter so cold it acts like one giant atom! This is a Bose–Einstein condensate (BEC). Scientists Albert Einstein and Satyendra Nath Bose predicted it in 1925. It happens when certain particles (called bosons) are cooled almost to absolute zero (about -273.15 degrees Celsius or -459.67 degrees Fahrenheit).
At this extreme cold, a large number of particles suddenly fall into the lowest possible energy state. They start to act together as a single wave. This is called "condensation" because it's like water vapor condensing into liquid.
In 1937, scientists found that helium-4 becomes a superfluid at very low temperatures. A superfluid has zero viscosity, meaning it flows without any friction. It can even "climb" out of its container! It also has perfect thermal conductivity, so no temperature differences can exist within it. These amazing properties are because helium-4 atoms form a Bose-Einstein condensate.
The first BEC in a gas was made in 1995. This was done by research groups led by Eric Cornell and Carl Wieman with rubidium atoms. Wolfgang Ketterle also made one with sodium atoms that same year.
Superconductors: Perfect Electricity Flow
Imagine an electric wire with no resistance at all! Superconductors are materials that have zero electrical resistance. This means electricity can flow through them perfectly, without losing any energy.
This special state exists at very low temperatures. When a superconductor is heated above a certain temperature, its resistance suddenly returns.
Superconductors also push out all magnetic fields from their inside. This is called the Meissner effect. Superconducting magnets are used in magnetic resonance imaging (MRI) machines.
Superconductivity was discovered in 1911. For a long time, it was only known in some metals at extremely cold temperatures (below 30 Kelvin). But in 1986, scientists found "high-temperature superconductivity" in certain ceramics. These can superconduct at much warmer, though still very cold, temperatures (up to 164 Kelvin).
A fermionic condensate is similar to a Bose-Einstein condensate. But it's made of different particles called fermions. Fermions usually can't be in the same quantum state. However, pairs of fermions can join up. These pairs then act like bosons and can form a condensate. Superconductors are an example of fermionic condensates.
Degenerate Matter: Stars Under Pressure
Under extreme pressure, like in the cores of dead stars, matter can change into exotic states. These are called degenerate matter. They are supported by special quantum rules.
In degenerate matter, particles are packed so tightly that they can't move much even when heated. This is because there are no more "slots" for them to move into. This is why degenerate stars become incredibly dense.
Electron-degenerate matter is found in white dwarf stars. Here, electrons are still linked to atoms. But they can move between nearby atoms.
Neutron-degenerate matter is found in neutron stars. Gravity is so strong that electrons are forced into protons. This creates a super-dense mix of neutrons. Planets like Jupiter and brown dwarfs also have cold degenerate matter in their cores.
Quark Matter: Tiny Particles Freed
In normal matter, tiny particles called quarks are always stuck together. They form larger particles like protons and neutrons. This is due to the strong force.
Quark matter is a state where the strong force is overcome. Quarks become free and can move around. This happens at extremely high densities or temperatures. Scientists can briefly create this state in powerful particle accelerators. This lets them study how quarks behave.
One type is quark–gluon plasma. In this super-hot state, quarks are free. They move in a "sea" of gluons. Gluons are the particles that carry the strong force. This is like electrons being freed from atoms in a normal plasma. Scientists first detected quark-gluon plasma in 2000. Unlike regular plasma, which acts like a gas, quark-gluon plasma acts more like a liquid.
Supersolids: Flowing Solids
Imagine a solid that can flow without friction! A supersolid is a material that is ordered like a crystal. But it also has the amazing properties of a superfluid.
Like a superfluid, a supersolid can move without any friction. Yet, it keeps its rigid, solid shape. This combination of properties makes supersolids a very unique state of matter.
Images for kids
See also
 In Spanish: Estado de agregación de la materia para niños
In Spanish: Estado de agregación de la materia para niños
- Chemical equation § State of matter
- Classical element
- Condensed matter physics
- Cooling curve
- Hidden states of matter
- List of states of matter
- Supercooling
- Superheating